Effects of 3-indoleacrylic acid on alleviating lipopolysaccharide-induced liver inflammatory damage in laying hens
- PMID: 40446688
- PMCID: PMC12166871
- DOI: 10.1016/j.psj.2025.105307
Effects of 3-indoleacrylic acid on alleviating lipopolysaccharide-induced liver inflammatory damage in laying hens
Abstract
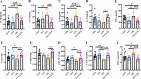
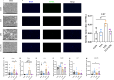
This study investigates the effects of 3-indoleacrylic acid (IAA), a bacterial metabolite of tryptophan, on LPS-induced liver injury in laying hens. The aim is to explore the potential hepatoprotective mechanisms of IAA, particularly through the modulation of inflammatory and antioxidant pathways. A total of 48, 24-week-old No. 6 Jingfen laying hens were randomly divided into four groups of 12 hens each: the CON group (basal diet), IAA group (basal diet + 150 mg/kg IAA), LPS group (basal diet), and IAA+LPS group (basal diet + 150 mg/kg IAA). The feeding period lasted 8 weeks, and at the end of the 8th week, the laying hens in the LPS and IAA+LPS groups were intraperitoneally injected with 0.5 mg/kg LPS. The four groups were uniformly slaughtered and sampled 12 h after LPS injection. The expression of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6) was significantly alleviated (P < 0.05), and antioxidant enzymes (T-SOD, CAT, GSH-PX) were significantly elevated (P < 0.05) in the serum and liver of the IAA+LPS group. Histological analysis revealed reduced hepatocyte necrosis and inflammation in the IAA-treated groups (P < 0.05). Additionally, the activation of MAPK and Toll-like receptor (TLR) signaling pathways was modulated through transcript analyses, thereby reducing inflammation and oxidative stress. In summary, IAA effectively mitigates liver inflammation and oxidative stress through the regulation of MAPK and TLR signaling pathways, providing a promising strategy for improving liver health in hens. This study represents the first report on the application of IAA in poultry, demonstrating its potential as a novel therapeutic agent for alleviating liver injury.
Keywords: 3-Indoleacrylic acid; Lay hens; Liver inflammatory damage; MAPK signaling pathway; Oxidative stress.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Protective Effects of α-Linolenic Acid on Liver Inflammation and Oxidative Stress Induced by Lipopolysaccharide in Broilers.Anim Sci J. 2025 Jan-Dec;96(1):e70096. doi: 10.1111/asj.70096. Anim Sci J. 2025. PMID: 40776587
-
Alleviation of lipopolysaccharide-induced heart inflammation in poultry treated with carnosic acid via the NF-κB and MAPK pathways.J Anim Sci. 2025 Jan 4;103:skae373. doi: 10.1093/jas/skae373. J Anim Sci. 2025. PMID: 39657120
-
Evaluating the therapeutic effect of different forms of silymarin on liver status and expression of some genes involved in fat metabolism, antioxidants and anti-inflammatory in older laying hens.Vet Med Sci. 2024 Nov;10(6):e70025. doi: 10.1002/vms3.70025. Vet Med Sci. 2024. PMID: 39324876 Free PMC article.
-
Effects of Bacillus subtilis ZY1 on production performance, egg quality, serum parameters and intestinal health in laying hens.Poult Sci. 2025 Jul;104(7):105120. doi: 10.1016/j.psj.2025.105120. Epub 2025 Apr 1. Poult Sci. 2025. PMID: 40319583 Free PMC article.
-
Dietary resveratrol alleviates liver and intestinal injury in ducks under cage rearing system.Poult Sci. 2025 Aug;104(8):105330. doi: 10.1016/j.psj.2025.105330. Epub 2025 May 22. Poult Sci. 2025. PMID: 40449104 Free PMC article.
References
-
- Abo Ghanima M.M., Abd El-Hack M.E., Al-Otaibi A.M., Nasr S., Almohmadi N.H., Taha A.E., Jaremko M., El-Kasrawy N.I. Growth performance, liver and kidney functions, blood hormonal profile, and economic efficiency of broilers fed different levels of threonine supplementation during feed restriction. Poult. Sci. 2023;102 - PMC - PubMed
-
- Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host. Microbe. 2018;23:716–724. - PubMed
-
- Amarasiri R., Hyun J., Lee S.W., Kim J.I., Lee H.G., Ryu B., Jeon Y.J. Therapeutic potential of tryptophan metabolite indoleacrylic acid in inflammatory bowel disease: From cellular mechanisms to zebrafish stress-like behavior. Int. Immunopharmacol. 2025;149 - PubMed
-
- Bender M.J., McPherson A.C., Phelps C.M., Pandey S.P., Laughlin C.R., Shapira J.H., Medina Sanchez L., Rana M., Richie T.G., Mims T.S., et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186:1846–1862.e1826. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

