Mannose-modified hemocyanin enhances pathogen endocytosis by crustacean hemocytes
- PMID: 40447191
- PMCID: PMC12221358
- DOI: 10.1016/j.jbc.2025.110304
Mannose-modified hemocyanin enhances pathogen endocytosis by crustacean hemocytes
Abstract
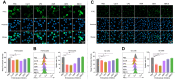
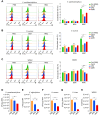
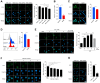
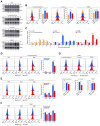
In crustaceans, hemolymph plasma contains more than 90% hemocyanin, whereas hemocytes have minimal levels, suggesting a regulated uptake mechanism. Here, we demonstrate that in Penaeus vannamei, hemocytes internalize plasma hemocyanin under normal conditions via phagocytosis, clathrin-mediated endocytosis, and micropinocytosis. This uptake is significantly enhanced during bacterial (Vibrio parahaemolyticus, Vibrio alginolyticus, Staphylococcus aureus, Streptococcus iniae) and viral (White spot syndrome virus) infections or upon stimulation with pathogen-associated molecular patterns. While post-translational modifications (PTMs) such as dephosphorylation, deacetylation, and mannosylation enhance hemocyanin's pathogen-binding affinity, only mannosylation promotes mannose receptor-mediated endocytosis for intracellular clearance, whereas dephosphorylation and deacetylation facilitate extracellular pathogen elimination. These findings reveal that hemocyanin functions beyond oxygen transport, acting as an immune effector that undergoes PTMs to enhance intracellular pathogen clearance.
Keywords: cellular immune response; crustaceans; endocytosis; hemocyanin; invertebrate; membrane trafficking; post-translational modification (PTM); protein translocation; receptor endocytosis.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
Hemocyanin controls Vibrio levels in hemolymph by modulating intracellular ROS via p38 MAPK.Virulence. 2025 Dec;16(1):2546066. doi: 10.1080/21505594.2025.2546066. Epub 2025 Aug 25. Virulence. 2025. PMID: 40852966 Free PMC article.
-
A CRP-like protein mediates antibacterial immunity in Pacific white shrimp, Litopenaeus vannamei.Int J Biol Macromol. 2025 Aug;320(Pt 2):145835. doi: 10.1016/j.ijbiomac.2025.145835. Epub 2025 Jul 7. Int J Biol Macromol. 2025. PMID: 40633872
-
Linear Ubiquitination of Hemocyanin Mediated by LUBEL Regulates Innate Immunity in Penaeus vannamei.Int J Mol Sci. 2025 May 26;26(11):5110. doi: 10.3390/ijms26115110. Int J Mol Sci. 2025. PMID: 40507921 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A comprehensive review on hemocyanin from marine products: Structure, functions, its implications for the food industry and beyond.Int J Biol Macromol. 2024 Jun;269(Pt 1):132041. doi: 10.1016/j.ijbiomac.2024.132041. Epub 2024 May 4. Int J Biol Macromol. 2024. PMID: 38705315 Review.
References
-
- Huang Y., Ren Q. Research progress in innate immunity of freshwater crustaceans. Dev. Comp. Immunol. 2020;104 - PubMed
-
- Mydlarz L.D., Jones L.E., Harvell C.D. Innate immunity environmental drivers and disease ecology of marine and freshwater invertebrates. Annu. Rev. Ecol. Evol. Syst. 2006;37:251–288.
-
- Osta M.A., Christophides G.K., Vlachou D., Kafatos F.C. Innate immunity in the malaria vector Anopheles gambiae:comparative and functional genomics. J. Exp. Biol. 2004;207:2551–2563. - PubMed
-
- Watson F.L., Püttmann-Holgado R., Thomas F., Lamar D.L., Hughes M., Kondo M., et al. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309:1874–1878. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

