Development and validation of the Immune Profile Score (IPS), a novel multiomic algorithmic assay for stratifying outcomes in a real-world cohort of patients with advanced solid cancer treated with immune checkpoint inhibitors
- PMID: 40447316
- PMCID: PMC12128404
- DOI: 10.1136/jitc-2024-011363
Development and validation of the Immune Profile Score (IPS), a novel multiomic algorithmic assay for stratifying outcomes in a real-world cohort of patients with advanced solid cancer treated with immune checkpoint inhibitors
Abstract
Background: Immune checkpoint inhibitors (ICIs) have transformed the oncology treatment landscape. Despite substantial improvements for some patients, the majority do not benefit from ICIs, indicating a need for predictive biomarkers to better inform treatment decisions.
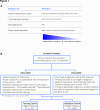
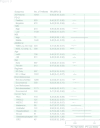
Methods: A de-identified pan-cancer cohort from the Tempus multimodal real-world database was used for the development and validation of the Immune Profile Score (IPS) algorithm leveraging Tempus xT (648 gene DNA panel) and xR (RNA sequencing) (N=1,707 development cohort; N=1,600 validation cohort). The cohort consisted of patients with advanced stage cancer with solid tumor carcinomas across 16 cancer types treated with any ICI-containing regimen as the first or second line of therapy. The IPS model was developed using a machine learning framework that includes tumor mutational burden (TMB) and 11 RNA-based biomarkers as features.
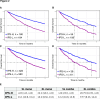
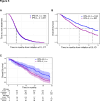
Results: IPS-High patients demonstrated significantly longer overall survival (OS) compared with IPS-Low patients (HR=0.45, 90% CI (0.40 to 0.52)). IPS was consistently prognostic in programmed death-ligand 1 (PD-L1) (positive/negative), TMB (High/Low), microsatellite status (microsatellite instability (MSI)-High), and regimen (ICI only/ICI+other) subgroups. Additionally, IPS remained significant in multivariable models controlling for TMB, MSI, and PD-L1, with IPS HRs of 0.49 (90% CI 0.42 to 0.56), 0.47 (90% CI 0.41 to 0.53), and 0.45 (90% CI 0.38 to 0.53), respectively. In an exploratory predictive utility analysis of the subset of patients (n=345) receiving first-line chemotherapy (CT) and second-line ICI, there was no significant effect of IPS for time to next treatment on CT in L1 (HR=1.06 (90% CI 0.88 to 1.29)). However, there was a significant effect of IPS for OS on ICI in L2 (HR=0.63 (90% CI 0.49 to 0.82)). A test of interaction was statistically significant (p<0.01).
Conclusions: Our results demonstrate that IPS is a generalizable multiomic biomarker that can be widely used clinically as a prognosticator of ICI-based regimens.
Keywords: Biomarker; Gene expression profiling - GEP; Immune Checkpoint Inhibitor; Next generation sequencing - NGS; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: ADZ, RE, YL, AJ, SWH, SM, BT, MGR, NP, BMM, AKS, MAT-L, HN, JG, KAB, CS, MMS, TT and EEWC are employees of and stockholders in Tempus AI, Inc. a for-profit company. Additionally, BMM, AKS, MMS, ADZ, RE, AJ, and KAB are named on patents related to work at Tempus; HN is an employee of Northwestern Medicine; and EEWC is an independent contractor for AVEO and Flamingo Therapeutics. TC is a co-founder of Gritstone Oncology and holds equity. TC holds equity in An2H. TC acknowledges grant funding from Bristol-Myers Squibb, AstraZeneca, Illumina, Pfizer, An2H, and Eisai. TC has served as an advisor for Bristol-Myers, MedImmune, Squibb, Illumina, Tempus, Eisai, AstraZeneca, and An2H. TC holds ownership of intellectual property on using tumor mutation burden to predict immunotherapy response, with pending patent, which has been licensed. SPP receives scientific advisory income from: Amgen, AstraZeneca, BeiGene, Bristol-Myers Squibb, Eli Lilly, Jazz, Genentech, Illumina, Merck, Pfizer, Zai Labs. SPP’s university receives research funding from: Amgen, AstraZeneca, A2bio, Bristol-Myers Squibb, Eli Lilly, Fate Therapeutics, Gilead, Iovance, Merck, Pfizer, and Roche/Genentech. MZ reports Institutionally-directed research funding from BMS and Exelixis; Advisory income from Pfizer, Exelixis, Janssen, Merck; and Honoraria from Adicet Bio and Arcus Bio. DA reports consulting or scientific advisory board support from Adlai Nortye, Boehringer Ingelheim, Cue Biopharma, Exelixis, Genmab, Inhibrx, Immunitas, Sanofi, Kura Oncology, Merck, Merck KGaA, Merus, Natco Pharma, Purple Biotech, Regeneron, Seagen, and TargImmune Therapeutics; travel support from Natco Pharma; leadership role on the NCCN practice guidelines and the Barnes Jewish Hospital Pharmacy and Therapeutics committee; research support from Tempus, Pfizer, Eli Lilly, Merck, Celgene/BMS, Novartis, AstraZeneca, Blueprint Medicine, Kura Oncology, Cue Biopharma, Cofactor Genomics, Debiopharm International, Inhibrx, ISA Therapeutics, Gilead Sciences, BeiGene, Roche, Vaccinex, Hookipa Biotech, Adlai Nortye USA, Epizyme, BioAtla, Boehringer Ingelheim, Calliditas Therapeutics, Genmab, Natco Pharma, Tizona Therapeutics, Erasca, Alentix, Seagen, Coherus, Takeda, Xilio, GSK, Johnson & Johnson, and Immunotep.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
