Neovascular pruning by IDO1 inhibitors can potentiate immunogenic cytotoxicity of ischemia-targeted agents to synergistically enhance anti-PD-1 responsiveness
- PMID: 40447318
- PMCID: PMC12128478
- DOI: 10.1136/jitc-2024-011398
Neovascular pruning by IDO1 inhibitors can potentiate immunogenic cytotoxicity of ischemia-targeted agents to synergistically enhance anti-PD-1 responsiveness
Abstract
Background: Strategies for deploying indoleamine 2,3-dioxygenase 1 (IDO1)-targeted therapies for use against cancer have focused on IDO1's role in promoting peripheral immune tolerance that shields tumors from effector T cells. However, preclinical investigation of both primary and metastatic tumor development in the lungs has uncovered a previously unappreciated role for IDO1 in directing a counterregulatory response to interferon (IFN)-γ that realigns the local inflammatory environment to promote tumor neovascularization. Understanding how to therapeutically leverage the ability of IDO1 inhibitors to subvert inflammatory neovascularization within the tumor microenvironment has potential ramifications for future clinical development of these compounds.
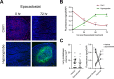
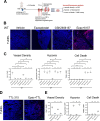
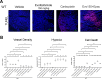
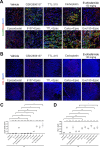
Methods: Pulmonary metastases seeded by orthotopically implanted 4T1 breast carcinoma cells were evaluated by confocal microscopy for the impact of both genetic and pharmacological IDO1 inhibition, alone or in combination with ischemia-directed cytotoxic agents, on markers of blood vessel density, hypoxia and cell death. Tumor immunogenicity and programmed death-ligand 1 (PD-L1) elevation were also evaluated. Quantitative analysis of these results was used to guide combinatorial treatment regimen development.
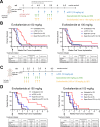
Results: Inhibiting IDO1 activity resulted in reduced neovascular density and elevated hypoxia in pulmonary metastases for which host IFN-γ was essential while adaptive immunity was dispensable. The tumors were consequently sensitized to the cytotoxic activity of ischemia-targeted agents including the protein kinase R-like endoplasmic reticulum kinase (PERK) inhibitor GSK2656157, the dithiol oxidative antimetabolite TTL-315, and the hypoxia-activated prodrug evofosfamide. Evofosfamide provoked the greatest degree of immunogenic cell death, while hypoxia, among other stressors, induced PD-L1. Based on this information, synergistic improvement in median survival was demonstrated in mice with established lung metastases through combined administration of anti-programmed cell death protein-1 (PD-1) antibody with evofosfamide and the IDO1 inhibitor epacadostat.
Conclusions: Improving therapeutic outcomes for patients with lung tumors, arising either as primary lesions or metastatic colonies, is of vital clinical importance. Building on preclinical evidence for IDO1's role in promoting inflammatory neovascularization of lung tumors, this study demonstrates how the intratumoral ischemic stress elicited by IDO1 inhibition can potentiate the immunogenic cytotoxicity of ischemia-targeted agents to effectively leverage immune checkpoint blockade responsiveness to confer a synergistic survival benefit. These findings provide a novel perspective on how IDO1 inhibitors can impact tumor biology and open up new possibilities for therapeutic applications.
Keywords: Combination therapy; Immune Checkpoint Inhibitor; Indoleamine 2, 3-dioxygenase - IDO; Lung Cancer; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: AJM is the recipient of financial support through sponsored research and consulting agreements with IO Biotech, Inc. AJM is also a co-inventor in the discovery and development of IDO1 inhibitor technologies patented by the Lankenau Institute for Medical Research and licensed to Augen Therapeutics, Inc., a private company for which he serves as a scientific advisor. SD is employed by Wuxi Advanced Therapeutics, Inc. MTH is Executive Chairman and CEO of Telesis Therapeutics, Inc. GCP is a co-inventor in the discovery and development of IDO1 inhibitor technologies patented by the Lankenau Institute for Medical Research and licensed to Augen Therapeutics, Inc., a private company for which he serves as a scientific advisor. The remaining authors declare no competing interests.
Figures


References
-
- Smith C, Chang MY, Parker KH, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2:722–35. doi: 10.1158/2159-8290.CD-12-0014. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
