Effect of elexacaftor/tezacaftor/ivacaftor on systemic inflammation in cystic fibrosis
- PMID: 40447326
- PMCID: PMC12421123
- DOI: 10.1136/thorax-2024-222242
Effect of elexacaftor/tezacaftor/ivacaftor on systemic inflammation in cystic fibrosis
Abstract
Background: Despite significant clinical improvements, there is evidence of persisting airway inflammation in people with cystic fibrosis (CF) established on elexacaftor/tezacaftor/ivacaftor (ETI) therapy. As CF is a multi-system disease, systemic immune profiles can reflect local inflammation within the lungs and other organs. Understanding systemic inflammation after ETI therapy may reveal important translational insights. This study aims to profile systemic inflammatory changes and relate these to the well-documented improvements observed with ETI therapy.
Methods: We conducted a single-centre longitudinal study with 57 CF subjects initiating ETI therapy. All participants were Phe508del homozygous or Phe508del/minimal function. Blood samples were collected pre-ETI and 3-12 months post-therapy initiation. Analyses included mass spectrometry-based proteomics, a multiplex immunoassay, and flow cytometry for peripheral immune cell counts and phenotype. Controls samples were provided by 29 age-matched healthy controls.
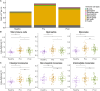
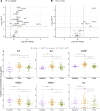
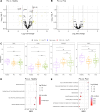
Results: Systemic inflammation reduced with ETI therapy; however, the immune profile remained distinct from healthy controls. ETI reduced neutrophil counts and was associated with a more mature, less inflammatory phenotype, as well as a shift towards an immune resolving state associated with increased CD206 expression. Cytokines known to influence neutrophil levels reduced with therapy. Despite ETI therapy, neutrophil and monocyte counts remained elevated compared with healthy controls. There was no obvious association between the ETI-related improvements in systemic inflammation and lung function.
Conclusions: Patients with CF showed evidence of persisting systemic inflammation despite ETI therapy, which may have long-term potentially adverse effects on respiratory and other organ systems.
Keywords: Cystic Fibrosis; Neutrophil Biology.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: PB declares speaker fees (Vertex and Chiesi) and Advisory board participation (Vertex, Boehringer Ingelheim and Insmed).
Figures


References
-
- Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940–8. doi: 10.1016/S0140-6736(19)32597-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
