An Observational Study of Cardiovascular Outcomes of Tirzepatide vs Glucagon-Like Peptide-1 Receptor Agonists
- PMID: 40447342
- PMCID: PMC12235410
- DOI: 10.1016/j.jacadv.2025.101740
An Observational Study of Cardiovascular Outcomes of Tirzepatide vs Glucagon-Like Peptide-1 Receptor Agonists
Abstract
Background: While cardiovascular benefits of tirzepatide, a glucose-dependent insulinotropic peptide/glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes mellitus (T2DM), and its comparative effectiveness vs glucagon-like peptide-1 receptor agonists (GLP-1RAs) is studied in randomized controlled trials, real-world outcomes may provide critical insights.
Objectives: The purpose of this study was to examine the cardiovascular benefits of tirzepatide vs GLP-1RA in people living with overweight or obesity, with T2DM, age ≥40 years, and pre-existing ischemic heart disease (IHD).
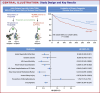
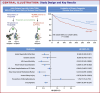
Methods: A retrospective cohort analysis of de-identified, aggregate patient data from the TriNetX research network was conducted. People with T2DM, age ≥40 years, pre-existing IHD, and body mass index ≥25 kg/m2 receiving either tirzepatide or GLP-1RA were identified and divided into 2 groups (tirzepatide vs GLP-1RA). After propensity score matching, Cox-proportional HRs were used to compare efficacy and safety outcomes during 1-year follow-up.
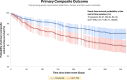
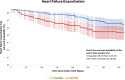
Results: Among 47,719 adults, 753 received tirzepatide, and 46,966 were on GLP-1RA. After propensity score matching, each group had 751 adults (mean age 59.9 ± 8.9 years, 46.5% females, 74.8% White adults in the tirzepatide group). Treatment with tirzepatide was associated with lower primary composite outcomes of acute myocardial infarction, ischemic stroke, and all-cause mortality (HR: 0.60, 95% CI: 0.43-0.84, P < 0.001). Individually, acute myocardial infarction (HR: 0.59, 95% CI: 0.38-0.91) and all-cause mortality (HR: 0.35, 95% CI: 0.14-0.88, P = 0.001) were also found to be favorable in the tirzepatide group.
Conclusions: Tirzepatide use is associated with better outcomes in adults aged 40 years or older with T2DM, body mass index ≥25 kg/m2, and pre-existing IHD.
Keywords: GLP-1 receptor agonists; cardiovascular outcomes; tirzepatide.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding support and author disclosures Dr Fonarow reports having consulted for Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr Bhatt discloses the following relationships: is in the advisory board of Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, and Stasys; is a part of Board of Directors at American Heart Association New York City, Angiowave (stock options), Bristol Myers Squibb (stock), DRS.LINQ (stock options), and High Enroll (stock); is a consultant for Broadview Ventures, GlaxoSmithKline, Hims, SFJ, and Youngene; is a part of Data Monitoring Committees at Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); received Honoraria from American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; is the Chair of the ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), WebMD (CME steering committees), Wiley (steering committee); other: Clinical Cardiology (Deputy Editor); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald's Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo. Dr Jhund reports speaker fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, Sun Pharmaceuticals, and Intas Pharmaceuticals; advisory board fees from AstraZeneca, Boehringer Ingelheim, and Novartis; research funding from AstraZeneca, Boehringer Ingelheim, and Analog Devices Inc; and is the director of Global Clinical Trial Partners. Dr Jhund's employer, the University of Glasgow, has been remunerated for clinical trial work from AstraZeneca, Bayer AG, Novartis, and Novo Nordisk. Dr Kosiborod has received research grants from AstraZeneca and Boehringer Ingelheim and has served as a consultant for Alnylam, AstraZeneca, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Esperion Therapeutics, Janssen, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Sanofi, and Vifor. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
References
-
- Sattar N., Lee M.M.Y., Kristensen S.L., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–662. doi: 10.1016/S2213-8587(21)00203-5. - DOI - PubMed
LinkOut - more resources
Full Text Sources

