Developmental Olfactory Dysfunction and Abnormal Odor Memory in Immune-Challenged Disc1+/- Mice
- PMID: 40447445
- PMCID: PMC12178285
- DOI: 10.1523/JNEUROSCI.1007-24.2025
Developmental Olfactory Dysfunction and Abnormal Odor Memory in Immune-Challenged Disc1+/- Mice
Abstract
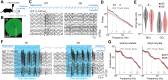
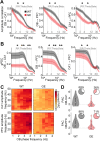
Neuronal activity in the olfactory bulb (OB) drives coordinated activity in the hippocampal-prefrontal network during early development. Inhibiting OB output in neonatal mice disrupts functional development of the hippocampal formation as well as cognitive abilities. These impairments manifest early in life and resemble dysfunctions of the hippocampus and the prefrontal cortex that have been linked to neuropsychiatric disorders. Thus, we investigated OB activity during early development in a disease mouse model and asked whether activity disruptions might contribute to the dysfunctional development of the hippocampal-prefrontal network. We addressed this question by combining in vivo electrophysiology with behavioral assessment of immune-challenged Disc1+/- mice of both sexes that mimic the dual genetic-environmental etiology of neuropsychiatric disorders. In wild-type mice, we found high DISC1 expression levels in OB projection neurons during development. Furthermore, neuronal and network activity in the OB and the drive from the bulb to the hippocampal-prefrontal network were reduced in immune-challenged Disc1+/- mice during early development. This early deficit did not affect odor-evoked activity and odor perception but resulted in impaired long-term odor memory. We propose that reduced spontaneous activity in the developing OB might contribute to altered maturation of the hippocampal-prefrontal network, leading to memory impairment in immune-challenged Disc1+/- mice.
Keywords: Disc1; development; hippocampus; neuropsychiatric; olfaction; prefrontal cortex.
Copyright © 2025 Parbst et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

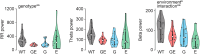




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
