Distinct brain atrophy progression subtypes underlie phenoconversion in isolated REM sleep behaviour disorder
- PMID: 40447483
- PMCID: PMC12177146
- DOI: 10.1016/j.ebiom.2025.105753
Distinct brain atrophy progression subtypes underlie phenoconversion in isolated REM sleep behaviour disorder
Abstract
Background: Synucleinopathies include a spectrum of disorders varying in features and severity, including idiopathic/isolated REM sleep behaviour disorder (iRBD), Parkinson's disease (PD), and dementia with Lewy bodies (DLB). Distinct brain atrophy patterns may already be seen in iRBD; however, how brain atrophy begins and progresses remains unclear.
Methods: A multicentric cohort of 1276 participants (451 polysomnography-confirmed iRBD, 142 PD with probable RBD, 87 DLB, and 596 controls) underwent T1-weighted MRI and longitudinal clinical assessments. Brain atrophy was quantified using vertex-based cortical surface reconstruction and volumetric segmentation. The unsupervised machine learning algorithm, Subtype and Stage Inference (SuStaIn), was used to reconstruct spatiotemporal patterns of brain atrophy progression.
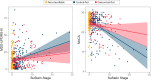
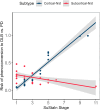
Findings: SuStaIn identified two distinct subtypes of brain atrophy progression: 1) a "cortical-first" subtype, with atrophy beginning in the frontal lobes and involving the subcortical structures at later stages; and 2) a "subcortical-first" subtype, with atrophy beginning in the limbic areas and involving cortical structures at later stages. Both cortical- and subcortical-first subtypes were associated with a higher rate of increase in MDS-UPDRS-III scores over time, but cognitive decline was subtype-specific, being associated with advancing stages in patients classified as cortical-first but not subcortical-first. Classified patients were more likely to phenoconvert over time compared to stage 0/non-classified patients. Among the 88 patients with iRBD who phenoconverted during follow-up, those classified within the cortical-first subtype had a significantly increased likelihood of developing DLB compared to PD, unlike those classified within the subcortical-first subtype.
Interpretation: There are two distinct atrophy progression subtypes in iRBD, with the cortical-first subtype linked to an increased likelihood of developing DLB, while both subtypes were associated with worsening parkinsonian motor features. This underscores the potential utility of subtype identification and staging for monitoring disease progression and patient selection for trials.
Funding: This study was supported by grants to S.R. from Alzheimer Society Canada (0000000082) and by Parkinson Canada (PPG-2023-0000000122). The work performed in Montreal was supported by the Canadian Institutes of Health Research (CIHR), the Fonds de recherche du Québec - Santé (FRQS), and the W. Garfield Weston Foundation. The work performed in Oxford was funded by Parkinson's UK (J-2101) and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The work performed in Prague was funded by the Czech Health Research Council (grant NU21-04-00535) and by The National Institute for Neurological Research (project number LX22NPO5107), financed by the European Union - Next Generation EU. The work performed in Newcastle was funded by the NIHR Newcastle BRC based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University. The work performed in Paris was funded by grants from the Programme d'investissements d'avenir (ANR-10-IAIHU-06), the Paris Institute of Neurosciences - IHU (IAIHU-06), the Agence Nationale de la Recherche (ANR-11-INBS-0006), Électricité de France (Fondation d'Entreprise EDF), the EU Joint Programme-Neurodegenerative Disease Research (JPND) for the Control-PD Project (Cognitive Propagation in Prodromal Parkinson's disease), the Fondation Thérèse et René Planiol, the Fonds Saint-Michel; by unrestricted support for research on Parkinson's disease from Energipole (M. Mallart) and the Société Française de Médecine Esthétique (M. Legrand); and by a grant from the Institut de France to Isabelle Arnulf (for the ALICE Study). The work performed in Sydney was supported by a Dementia Team Grant from the National Health and Medical Research Council (#1095127). The work performed in Cologne was funded by the Else Kröner-Fresenius-Stiftung (grant number 2019_EKES.02), the Köln Fortune Program, Faculty of Medicine, University of Cologne, and the "Netzwerke 2021 Program (Ministry of Culture and Science of Northrhine Westphalia State). The work performed in Aarhus was supported by funding from the Lundbeck Foundation, Parkinsonforeningen (The Danish Parkinson Association), and the Jascha Foundation.
Keywords: Dementia with Lewy bodies; MRI; Machine learning; Parkinson's disease; REM sleep behaviour disorder; Subtyping.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Outside the submitted work, Stephen Joza received support for attending meetings and/or travel from the American Academy of Neurology and Parkinson's Canada. Jean-François Gagnon received funding from the NIH/NIA. Ronald B. Postuma received grants from the CIHR, Michael J. Fox Foundation, NIH, Roche Diagnostics, and the Weston Foundation. He received consulting fees from Novartis, Eisai, Merck, Vaxxinity, BMS, Ventus, Korro, Vanqua, Roche, Regeneron, Helicon, Epic, and Clinilabs. He holds leadership roles with Parkinson Canada, the Michael J. Fox Foundation, MDS, Movement Disorders journal, and the RBD Study Group. Alain Dagher received travel support from the Michael J. Fox Foundation. Johannes C. Klein receives salary support from the NIHR Oxford Health Clinical Research Facility and the NIHR Oxford BRC, speaker honoraria from Merz, Ipsen, and AbbVie, and travel reimbursement from Merz and Ipsen. Michele Hu received consulting fees from Lundbeck, ESCAPE Bio, Evidera, Manus Neurodynamica, Biogen MA, CuraSen Therapeutics, Roche, JAZZ Pharma, and Aventis Pharma. She received honoraria and support for attending meetings from the International Movement Disorders Society, the 10th Singapore International Parkinson Disease and Movement Disorder Symposium, and the World Parkinson Congress. She holds a patent for predicting striatal dopamine levels via smartphone, serves on advisory boards and DSMBs including the Exenatide-PD3 Trial and ISAP Trial Steering Committee, is Treasurer of the ABN MDSIG, a shareholder and advisory founder of NeuHealth Digital Ltd. John T. O'Brien received consulting fees from Biogen and acted as a consultant for Roche, GE Healthcare, and Okwin. He received honoraria for lectures from GE Healthcare, serves on advisory boards or DSMBs for TauRx and Novo Nordisk, chairs the Research Strategy Council of the UK Alzheimer's Society, and received research support from Avid/Lilly, Merck, UCB, and Alliance Medical. Paul C. Donaghy received grants or contracts, paid to his institution, from Alzheimer's Research UK, the Michael J. Fox Foundation, the Alzheimer's Society, and GE Healthcare, and received an honorarium for a lecture at the Lewy Body Masterclass (paid to his institution). Jean-Christophe Corvol received grants/contracts from the Paris Brain Institute, ANR, and AXA Foundation (paid to institution), consulting fees from Roche, Servier, UCB, Ferrer, Alzprotect, iRegene, and Bayer, and serves on the Servier advisory board. Richard Camicioli serves (unpaid) on the Research and Scientific Advisory Board of Parkinson Canada. Howard Chertkow is the Scientific Director of CCNA (unpaid) and principal investigator or co-investigator on major research grants including from CIHR ($20.3M, 2024–29), Alzheimer's Society of Canada, BrightFocus, and NIH. He has also received funding for multi-site clinical trials sponsored by IntelGenX, Alector, Eli Lilly, Biogen, Hoffman LaRoche, and Anavex. He serves on advisory boards for Lilly and Eisai (personal payment). Simon Lewis received travel support from the International Parkinson's and Movement Disorder Society as a member of their Congress Scientific Program Committee (2022–2025). He holds leadership roles on editorial boards (Translational Neurodegeneration, Journal of Parkinson's Disease, Movement Disorders, Parkinsonism and Related Disorders, Journal of Neurology) and various international MDS working groups. Elie Matar received honoraria from CSL Seqirus and the International Parkinson's and Movement Disorders Society for presentations on non-motor and cognitive symptoms in Parkinson's. Dario Arnaldi received honoraria for lectures from Idorsia, Italfarmaco, PIAM, and Bruno. Beatrice Orso received a research grant from GE Healthcare. Shady Rahayel received grant support and travel reimbursement from the Michael J. Fox Foundation. Aline Delva, Christina Tremblay, Andrew Vo, Marie Filiatrault, Max Tweedale, John-Paul Taylor, Michael Firbank, Alan Thomas, Petr Dusek, Stanislav Marecek, Zsoka Varga, Stephane Lehericy, Isabelle Arnulf, Marie Vidailhet, Kaylena A. Ehgoetz Martens, Lachlan Churchill, Michael Sommerauer, Sinah Röttgen, Per Borghammer, Karoline Knudsen, Allan K. Hansen, Pietro Mattioli, Luca Roccatagliata, and Oury Monchi report no conflicts of interest.
Figures




References
-
- Spillantini M.G., Schmidt M.L., Lee V.M.Y., Trojanowski J.Q., Jakes R., Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. - PubMed
-
- Darweesh S.K.L., Verlinden V.J.A., Stricker B.H., Hofman A., Koudstaal P.J., Ikram M.A. Trajectories of prediagnostic functioning in Parkinson's disease. Brain. 2017;140(2):429–441. - PubMed
-
- Berg D., Borghammer P., Fereshtehnejad S.M., et al. Prodromal Parkinson disease subtypes — key to understanding heterogeneity. Nat Rev Neurol. 2021;17(6):349–361. - PubMed
-
- Joza S., Hu M.T., Jung K.Y., et al. Progression of clinical markers in prodromal Parkinson's disease and dementia with Lewy bodies: a multicentre study. Brain. 2023;146(8):3258–3272. - PubMed
-
- Rahayel S., Tremblay C., Vo A., et al. Brain atrophy in prodromal synucleinopathy is shaped by structural connectivity and gene expression. Brain. 2022;145(9):3162–3178. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

