The a subunit isoforms of V-ATPase are involved in glucose-dependent trafficking of insulin granules
- PMID: 40447629
- PMCID: PMC12125285
- DOI: 10.1038/s41598-025-02997-7
The a subunit isoforms of V-ATPase are involved in glucose-dependent trafficking of insulin granules
Abstract
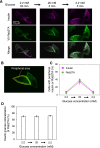
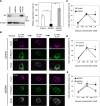
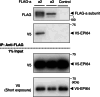
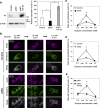
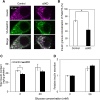
In pancreatic β cells, insulin granules move toward the plasma membrane to secrete insulin upon glucose stimulation, but the amount of secreted insulin is only a small portion of the total, and many granules do not release insulin. Here, using MIN6 cells derived from mouse pancreatic β cells, we observed that granules that moved toward the plasma membrane returned to the inner area after the stimulation was removed. This back-and-forth trafficking is likely important for strict regulation of insulin secretion in response to the blood glucose level. However, the mechanism was largely unknown. We found that "back" (inward) and "forth" (outward) trafficking was reduced in cells with knockdown of the a2 and a3 subunit isoforms of the proton pump V-ATPase, respectively. Interestingly, the amount of secreted insulin was increased in a2 knockdown cells. Both a2 and a3 interacted with GDP-bound form Rab27A, a member of the Rab small GTPase family that regulates insulin secretion. These results indicate that a2 and a3 are involved in back-and-forth trafficking of insulin granules, respectively. The a subunit isoforms of V-ATPase seem to determine the direction of insulin granule trafficking dependent on the glucose level.
Keywords: Insulin secretion; Membrane trafficking; Rab27A; V-ATPase; a subunit isoforms.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

