Improved aerobic capacity in a randomized controlled trial of noncombustible nicotine and tobacco products
- PMID: 40447739
- PMCID: PMC12125353
- DOI: 10.1038/s41598-025-03904-w
Improved aerobic capacity in a randomized controlled trial of noncombustible nicotine and tobacco products
Abstract
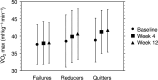
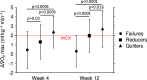
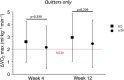
Smoking negatively impacts aerobic capacity, primarily by reducing V̇O2max, the gold standard measure of cardiorespiratory fitness. While smoking cessation is known to improve vascular function, exercise performance, and oxygen uptake, its specific impact on V̇O2max remains underexplored. Specifically, no research has yet evaluated V̇O2max changes following a switch to electronic cigarettes (ECs) or heated tobacco products (HTPs). This is a secondary analysis of the CEASEFIRE trial, a 12-weeks randomized controlled switching trial comparing the impact of ECs or HTPs on changes in smoking behaviour. The trial offers a unique opportunity to prospectively examine the relationship between smoking behavior and aerobic capacity, and to examine-for the first time-the specific impact of exclusive EC or HTP use on V̇O2max. Changes in VO₂max were analized across three smoking phenotypes: continuous smokers, those who reduced smoking, and those who abstained from smoking Additionally, VO2max was also evaluated specifically in participants who completely abstained from smoking tobacco cigarettes, evaluating outcomes in exclusive EC and HTP users. Quitters showed the greatest improvement in VO2max at both week 4 (2.4 ± 1.7 mL kg-1 min-1) and week 12 (2.7 ± 1.9 mL kg-1 min-1). Reducers also exhibited significant VO2max increases (1.3 ± 1.9 mL kg-1 min-1 at week 4: 1.9 ± 1.8 mL kg-1 min-1 at week 12), while Failures (i.e. those who continued smoking) showed no change. Exclusive use of EC and HTP resulted in statistically significant and clinically relevant improvements in V̇O2max. Compared to baseline, V̇O2max significantly increased at week 4 (EC: 38.4 ± 5.9 to 41.0 ± 6.1 mL kg-1 min-1; HTP: 39.2 ± 6.7 to 41.4 ± 6.4 mL kg-1 min-1, both p < 0.0001) and week 12 (EC: 38.4 ± 5.9 to 41.4 ± 6.3; HTP: 39.2 ± 6.7 to 41.6 ± 6.5 mL kg-1 min-1, both p < 0.0001). No significant differences between EC and HTP were observed at either time point. Rapid improvements in V̇O2max can happen when healthy smokers switch to exclusive use of ECs or HTPs. These findings reinforce the potential cardiorespiratory benefits of smoking cessation and harm reduction strategies.
Keywords: Aerobic capacity; Heated tobacco products; Smoking cessation; V̇O2max; e-cigarettes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: RP is full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same University. He has received grants from U-BIOPRED and AIR-PROM, Integral Rheumatology & Immunology Specialists Network (IRIS), Foundation for a Smoke Free World, Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, Merk Sharp & Dohme, Boehringer Ingelheim, Novartis, Arbi Group Srl., Duska Therapeutics, Forest Laboratories, Ministero dell Universita’ e della Ricerca (MUR) Bando PNRR 3277/2021 (CUP E63C22000900006) and 341/2022 (CUP E63C22002080006), funded by NextGenerationEU of the European Union (EU), and the ministerial grant PON REACT-EU 2021 GREEN- Bando 3411/2021 by Ministero dell Universita’ e (MUR) – PNRR EU Community. He is founder of the Center for Tobacco Prevention and Treatment (CPCT) at the University of Catania and of the Center of Excellence for the Acceleration of Harm Reduction at the same university. He receives consultancy fees from Pfizer, Boehringer Ingelheim, Duska Therapeutics, Forest Laboratories, CV Therapeutics, Sermo Inc., GRG Health, Clarivate Analytics, Guidepoint Expert Network, and GLG Group. He receives textbooks royalties from Elsevier. He is also involved in a patent application for ECLAT Srl. He is a pro bono scientific advisor for Lega Italiana Anti Fumo (LIAF) and the International Network of Nicotine Consumers Organizations (INNCO); and he is Chair of the European Technical Committee for Standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4). All other authors declare no conflict of interests.
Figures
References
-
- Montoye, H. J., Gayle, R. & Higgins, M. Smoking habits, alcohol consumption and maximal oxygen uptake. Med. Sci. Sports Exerc.12(5), 316–321 (1980). - PubMed
-
- Sengbusch, J. R., Tiernan, D. L., Tamulevicius, N. & Martinasek, M. P. The impact of smoking on maximum oxygen uptake. Respir. Care66(5), 857–886 (2021). - PubMed
-
- Cooper, K. H., Gey, G. O. & Bottenberg, R. A. Effects of cigarette smoking on endurance performance. JAMA203(3), 189–192 (1968). - PubMed
-
- Conway, T. L. & Cronan, T. A. Smoking, exercise, and physical fitness. Prev. Med.21(6), 723–734 (1992). - PubMed
-
- Suminski, R. R. et al. The effect of habitual smoking on measured and predicted VO2(max). J. Phys. Act Health.6(5), 667–673. 10.1123/jpah.6.5.667 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

