Patritumab deruxtecan in leptomeningeal metastatic disease of solid tumors: the phase 2 TUXEDO-3 trial
- PMID: 40447851
- PMCID: PMC12353872
- DOI: 10.1038/s41591-025-03744-1
Patritumab deruxtecan in leptomeningeal metastatic disease of solid tumors: the phase 2 TUXEDO-3 trial
Abstract
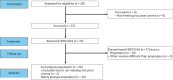
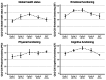
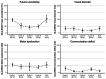
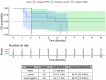
Leptomeningeal metastatic disease (LMD) is a severe complication of solid cancers with poor outcomes and limited treatment options. The antibody-drug conjugate patritumab deruxtecan (HER3-DXd) demonstrated efficacy in breast and lung cancers, and HER3 is involved in central nervous system metastases, particularly in parenchymal colonization. In this study, we investigated HER3-DXd efficacy and safety in patients with LMD in cohort 3 of the TUXEDO-3 phase 2 trial. Key eligibility criteria included age ≥18 years, treatment-naive LMD or LMD progressing after radiotherapy from any solid tumor and Eastern Cooperative Oncology Group performance status of 0-2. Between January and July 2024, 20 evaluable patients (nine with type I and 11 with type II LMD) were accrued and received HER3-DXd 5.6 mg kg-1 intravenously every 3 weeks. Main primary tumor types included breast (60%) and lung (30%) cancers. Median follow-up time was 5.4 months. The primary endpoint was met with 65.0% patients alive after 3 months. The Kaplan-Meier-estimated 3-month and 6-month overall survival rates were 69.6% and 58.9%, respectively. Overall response rate was 11.1% for intracranial, 30.8% for extracranial and 26.3% for overall lesions. Clinical benefit rate was 50.0% for intracranial, 38.5% for extracranial and 47.4% for overall lesions. Neurological symptoms and quality of life remained stable or improved during study treatment. No new neurological adverse events were observed. The most common adverse events of any grade were anemia (nine (40.9%) patients, one (4.5%) grade ≥3), nausea (seven (31.8%) patients, no grade ≥3), neutropenia (six (27.3%) patients, three (13.6%) grade ≥3), diarrhea (six (27.3%) patients, one (4.5%) grade ≥3), asthenia (six (27.3%) patients, no grade ≥3) and thrombocytopenia and headache (five (22.7%) patients, one (4.5%) grade ≥3 each). TUXEDO-3 showed clinically relevant HER3-DXd activity in patients with LMD. ClinicalTrials.gov identifier: NCT05865990 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.P. declares honoraria (Bayer, Bristol Myers Squibb, Novartis, Gerson Lehrman Group, CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, AstraZeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dohme, Tocagen, Adastra, Gan & Leen Pharmaceuticals, Janssen, Servier, Miltenyi, Boehringer Ingelheim, Telix and Medscape); consulting/advisory role (Bayer, Bristol Myers Squibb, Novartis, Gerson Lehrman Group, CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, AstraZeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dohme, Tocagen, Adastra, Gan & Leen Pharmaceuticals, Janssen, Servier, Miltenyi, Boehringer Ingelheim, Telix and Medscape); and travel, accomodations and expenses (Bristol Myers Squibb, Novartis, Mundipharma, Servier, Miltenyi, Roche, Daiichi Sankyo, MEDSIR, Boehringer Ingelheim, Telix and Medscape). M.G. declares honoraria (Novartis, Gilead, AstraZeneca and Pfizer); personal support for attending meetings and/or travel (Roche, Pfizer, AstraZeneca and Gilead); and honoraria for advisory board participation (Gilead, Novartis, AstraZeneca and Pfizer). R.G. declares stock or other ownership (Novo Nordisk and Lilly); honoraria (Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, Merck Sharp & Dohme, Sandoz, AbbVie, Gilead, Daiichi Sankyo and Sanofi); consulting/advisory role (Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, Merck Sharp & Dohme, Amgen, Merck, Gilead, Daiichi Sankyo and Sanofi); research funding (Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, Merck Sharp & Dohme, Sandoz, AbbVie, Gilead and Daiichi Sankyo); and travel, accomodations and expenses (Roche, Amgen, Janssen, AstraZeneca, Novartis, Merck Sharp & Dohme, Celgene, Bristol Myers Squibb, AbbVie, Gilead and Daiichi Sankyo). A.L.-C. declares research support (Roche, Agendia, Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Gilead and Daiichi Sankyo); consulting/advisory role (Lilly, Roche, Pfizer and Novartis); speaker’s bureaus (Lilly, AstraZeneca, Merck Sharp & Dohme, Pfizer and Novartis); travel support (Roche, Pfizer, AstraZeneca, Steamline Therapeutics and Merck Sharp & Dohme); patents (‘Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy’, US 2019/0338368 A1); and stock or other ownership (MAJ3 Capital and Initia Research). M.V. declares speaker grants (Pfizer, Novartis, Roche, Merck Sharp & Dohme, Gilead, Seagen, Pierre Fabre, Eisai and Sanofi-Aventis) and investigation fund (Fundación Quirónsalud). J.C. declares consulting/advisory role (Roche, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Lilly, Merck Sharp & Dohme, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, Reveal Genomics, Scorpion Therapeutics, Expres2ion Biotechnologies, Jazz Pharmaceuticals, AbbVie, BridgeBio, BioNTech, Biocon, Circle Pharma, Delcath Systems and Hexagon Bio); honoraria (Roche, Novartis, Eisai, Pfizer, Lilly, Merck Sharp & Dohme, Daiichi Sankyo, AstraZeneca, Gilead and Steamline Therapeutics); research funding to institution (Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta Gmbh/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardant Health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Iqvia and Queen Mary University of London); stock (MAJ3 Capital and Leuko (relative)); travel, accommodations and expenses (Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, AstraZeneca, Gilead, Merck Sharp & Dohme and Steamline Therapeutics); and patents (‘Pharmaceutical combinations of a Pi3k inhibitor and a microtubule destabilizing agent’, WO 2014/199294A, and ‘Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy’, US 2019/0338368 A1). M.C., J.A.G., P.G.-A., C.J.-C. and J.R.-M. declare full-time employment at MEDSIR. M.V.-B. declares honoraria (Daichii Sankyo, GlaxoSmithKline and AstraZeneca); consulting/advisory role (Daichii Sankyo and AstraZeneca); speakers’ bureau (Novartis); and travel, accommodations and expenses (AstraZeneca). F.O. declares honoraria (Merck Sharp & Dohme); consulting/advisory role (Merck Sharp & Dohme); and travel, accommodation and expenses (Incyte Biosciences Austria). M.M. declares consulting/advisory role (Lilly, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer and Gilead); speakers’ bureau (Lilly, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer and Gilead); research funding (Daiichi Sankyo); and travel, accommodations and expenses (Merck Sharp & Dohme, Gilead, Novartis, Lilly and Daiichi Sankyo). A.-S.B. declares research support (Daiichi Sankyo and Roche); honoraria for lectures, consultation or advisory board participation (Roche, Bristol Myers Squibb, Merck, Daiichi Sankyo, AstraZeneca, CeCaVa, Seagen, Alexion and Servier); and travel support (Roche, Amgen and AbbVie). J.F. declares consulting/advisory role (Seagen and Novartis) and speakers’ bureau (Seagen and Sanova). T.F. declares honoraria (Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Roche, Sanofi, Merck, BI, Janssen, Lilly, Invios, Takeda, Amgen, GlaxoSmithKline and Pfizer); consulting/advisory role (Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Roche, Sanofi, Merck, BI, Janssen, Lilly, Invios, Takeda, Amgen, GlaxoSmithKline, BeiGene and Daiichi Sankyo); research funding (Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Roche, Sanofi, Merck, BI, Janssen, Lilly, Invios, Takeda, Amgen, GlaxoSmithKline and Nanobiotix); and travel, accommodations and expenses (Merck Sharp & Dohme, Merck and Roche). R.B. declares honoraria (AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Eisai and Lilly); consulting/advisory role (AstraZeneca, Daichi Sankyo, Eisai and Lilly); research funding (Daiichi Sankyo); and travel, accommodations and expenses (Merck Sharp & Dohme, Daiichi Sankyo, Novartis and Pfizer). J.G., J.J.G.-M., M.A. and M.R.-B. declare no conflicts of interest.
Figures





References
-
- Ozair, A. et al. Leptomeningeal metastatic disease: new frontiers and future directions. Nat. Rev. Clin. Oncol.22, 134–154 (2025). - PubMed
-
- Le Rhun, E. et al. Prolonged survival of patients with breast cancer-related leptomeningeal metastases. Anticancer Res.33, 2057–2063 (2013). - PubMed
-
- Bartsch, R., Jerzak, K. J., Larrouquere, L., Müller, V. & Le Rhun, E. Pharmacotherapy for leptomeningeal disease in breast cancer. Cancer Treat. Rev.122, 102653 (2024). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

