IL-36 signaling as a drug target in Crohn's disease patients with IL36RN mutations
- PMID: 40447918
- PMCID: PMC12254353
- DOI: 10.1038/s44321-025-00245-z
IL-36 signaling as a drug target in Crohn's disease patients with IL36RN mutations
Abstract
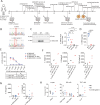
The IL-36 signaling pathway has recently been identified as a key regulator of intestinal homeostasis and inflammation. However, the role of mutations in the IL-36R signaling pathway in the pathogenesis of inflammatory bowel disease remains unclear. We here identified four Crohn's disease patients with heterozygous missense mutations in the IL-36 receptor antagonist (IL36RN, IL-36RA). Experimental overexpression and functional assays demonstrated that two identified mutations resulted in reduced expression of IL-36RA. In-depth immune profiling of one IL36RN-mutated patient revealed an increased response of PBMCs to IL-36 stimulation and elevated serum levels of IL-36-regulated cytokines. Administration of the IL-36R-blocking antibody spesolimab to this patient resulted in a reduction of intestinal inflammation and alterations in immune cell composition and function. Our findings indicate that pathogenic IL36RN mutations may contribute to the pathogenesis of Crohn's disease in a subset of patients and that inhibiting IL-36 signaling could offer a personalized therapeutic approach for these patients.
Keywords: Crohn’s Disease; Genetics; IL-36 Signaling; Personalized Therapy.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. BS received grant support by Pfizer, served as consultant for Abbvie, BMS, Boehringer, Endpoint Health, Falk, Galapagos, Gilead, Lilly, MSD, Pfizer, Takeda (BS served as representative of the Charité) and received speaker’s fees from Abbvie, BMS, CED Service GmbH, Falk, Ferring, Galapagos, Janssen, Lilly, Pfizer, Takeda (BS served as representative of the Charité). CW received grant support by Pfizer, served as a consultant for Pfizer and received speaker’s fees from Falk, Ferring, Janssen. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations in relation to the potential mention of BI substances. Boehringer Ingelheim had no role in the design, analysis or interpretation of the results in this study. Spesolimab was made available on the basis of an out-of-scope request.
Figures




References
-
- Bottcher C, Fernandez-Zapata C, Schlickeiser S, Kunkel D, Schulz AR, Mei HE, Weidinger C, Giess RM, Asseyer S, Siegmund B et al (2019) Multi-parameter immune profiling of peripheral blood mononuclear cells by multiplexed single-cell mass cytometry in patients with early multiple sclerosis. Sci Rep 9: 19471 - DOI - PMC - PubMed
-
- Boutet MA, Bart G, Penhoat M, Amiaud J, Brulin B, Charrier C, Morel F, Lecron JC, Rolli-Derkinderen M, Bourreille A et al (2016) Distinct expression of interleukin (IL)-36alpha, beta and gamma, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin Exp Immunol 184:159–173 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

