Immunogenicity and cellular response of a herpes zoster virus gEgI fusion protein adjuvanted with CpG-emulsion in mice
- PMID: 40448056
- PMCID: PMC12124028
- DOI: 10.1186/s12951-025-03423-w
Immunogenicity and cellular response of a herpes zoster virus gEgI fusion protein adjuvanted with CpG-emulsion in mice
Abstract
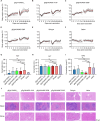
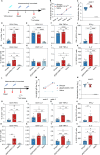
Herpes zoster (HZ), commonly known as shingles, arises from the reactivation of the latent varicella-zoster virus (VZV) when VZV-specific cellular immunity declines below a critical threshold necessary for viral suppression. The current leading vaccine, Shingrix, which incorporates the adjuvant AS01B with glycoprotein E (gE), has significantly contributed to HZ prevention but raises concerns regarding safety and accessibility. Addressing the need for safer and more accessible HZ vaccinations, we developed a vaccine comprising a fusion protein of glycoprotein E and I (gEgI), connected via a linker, targeting abundant B cell and CD4 T cell epitopes. Our study assessed the immunogenicity of the gE alone and the gEgI fusion protein in adult mice, revealing that gEgI prompts a more potent and comprehensive T cell response compared to gE alone. Furthermore, we introduced a composite adjuvant, an emulsion-type adjuvant combined with CpG1018 (XUA09C), which was shown to enhance both humoral and cellular immune responses beyond the capabilities of XUA09 with CpG alone. Comparative analyses demonstrated that the XUA09C-adjuvanted gEgI vaccine induces comparable antibody responses and significantly superior T cell responses relative to Shingrix in both adult, VZV-primed, and aged mice. Single-cell RNA sequencing highlighted that gEgI/XUA09C more effectively promotes early immune activation, B and T cell proliferation, and memory T cell augmentation compared to Shingrix. These findings position the XUA09C-adjuvanted gEgI as a promising candidate for further development in HZ vaccine strategies, potentially better serving the needs of the immunocompromised population.
Keywords: Adjuvant; CpG-emulsion; Glycoprotein E; Glycoprotein I; HZ vaccine; Single-cell RNA sequencing; Varicella-zoster virus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: T.L., S.L., S.Z., Y.Z., L.C., Y.G., T.C. and N.X. are inventors on a patent application (PCT/CN2024/073289) filed by the Xiamen University that covers gEgI fusion protein design described in this work.
Figures






References
-
- A AM: Varicella-Zoster virus. CLINMICROBIOLREV 1996:361–81.
-
- H. GD: Varicella-zoster virus DNA in human sensory ganglia. Nature 1983, 306. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

