USP24 upregulation stabilizes PKA-Cα to promote lipogenesis, inflammation, and fibrosis during MASH progression
- PMID: 40448065
- PMCID: PMC12125897
- DOI: 10.1186/s12929-025-01148-4
USP24 upregulation stabilizes PKA-Cα to promote lipogenesis, inflammation, and fibrosis during MASH progression
Abstract
Background: Ubiquitin-specific peptidase 24 (USP24), a deubiquitinating enzyme, regulates protein stability by removing ubiquitin. This study investigates the role of UPS24 in lipid metabolism, inflammation, and fibrosis. It also explores the effect of targeting USP24 on metabolic disorders, focusing on high-fat diet (HFD)-induced obesity and liver diseases.
Methods: This study utilized CRISPR/Cas9 to create functional knockout mice (USP24C1695A) and treated HFD-fed mice with USP24 inhibitor (USP24-i-101). The effects of USP24 inhibition or knockout on 3T3-L1 derived adipocytes, primary hepatocytes, hepatic stellate cells, and murine hepatocyte cell line AML12 (alpha mouse liver 12) cells were assessed with RNA-sequencing. Molecular mechanisms and the interaction between USP24 and PKA-Cα were studied with co-immunoprecipitation. Downstream signaling pathways involving CREB, SREBP1, PPARγ, and C/EBPβ, as well as USP24 role in liver inflammation and fibrosis, were studied using western blot and real-time PCR. Clinical and animal tissue samples were examined with immunohistochemistry to identify the correlations between USP24 and metabolic-associated liver diseases.
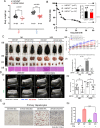
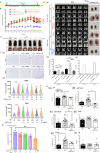
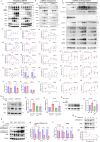
Results: Knockout or inhibition of USP24 reduced body weight, lipid accumulation, inflammation, and fibrosis in HFD-fed mice. The expression of genes related to lipogenesis, inflammation, and fibrosis was downregulated in USP24C1695A mice and those treated with USP24 inhibitor (USP24-i-101). USP24 inhibition decreased lipid droplet accumulation in adipocytes and hepatocytes, suppressed inflammation in hepatocytes and AML12 cells, and reduced fibrosis in hepatic stellate cells. Mechanistically, USP24 expression was upregulated by PKA activation during adipocyte differentiation, leading to increased PKA-Cα stability and CREB phosphorylation, which promoted lipogenic gene expression. Free fatty acids (FFA) increased USP24 expression, activating NF-κB and TGFβ pathways to induce inflammation (Cox2) and fibrosis (α-SMA). USP24 was highly expressed in patients with metabolic dysfunction-associated steatohepatitis (MASH) and correlated with Cox2 and α-SMA levels.
Keywords: Lipogenesis; MASH; PKA-Cα; USP24; p-CREB.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The IRB number is KMUHIRB-G(II)-20170013, which was approved by Institutional Review Board of Kaohsiung Medical University Hospital. Consent for publication: No applicable. Competing interests: The authors declare no potential competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

