Chimeric antigen receptors discriminate between tau and distinct amyloid-beta species
- PMID: 40448101
- PMCID: PMC12125761
- DOI: 10.1186/s12967-025-06572-6
Chimeric antigen receptors discriminate between tau and distinct amyloid-beta species
Abstract
Background: The lack of a definitive cure for Alzheimer's disease (AD) is fueling the search for innovative therapeutic strategies. Having revolutionized cancer immunotherapy, immune cell engineering with chimeric antigen receptors (CAR) is being explored to target AD. Whether CARs can recognize distinct amyloid-β (Aβ) species and tau neurofibrillary tangles (NFTs)-hallmark pathologies of AD-remains unclear.
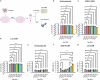
Methods: To investigate this, we engineered a series of CARs using single-chain fragment variable (scFv) derived from the variable light and heavy chains of antibodies tested in AD clinical trials. These included E2814 (E2814-CAR), targeting tau; Lecanemab (Lec-CAR) and Aducanumab (Adu-CAR), targeting Aβ; and Donanemab (Don-CAR) and Remternetug (Rem-CAR), targeting the truncated pyroglutamated Aβ species Aβp3-42. To evaluate CAR function, we utilized the murine DO11.10 CD4⁺ T-cell hybridoma line as a scalable and reproducible platform. CAR activation was assessed in response to tau preformed fibrils (PFFs), Aβ1-42 oligomer-enriched aggregates, and Aβp3-42 aggregates, using flow cytometry for CD69 expression and ELISA for IL-2 secretion. To validate this platform, we tested Adu-CAR in primary mouse CD4⁺ T cells treated with Aβ1-42 aggregates and assessed activation via flow cytometry for CD69 and CD25 expression.
Results: DO11.10 cells expressing E2814-CAR-but not Lec-CAR-responded to tau PFFs. In contrast, cells expressing Adu-CAR, and to a lesser extent Lec-CAR-but not E2814-CAR-responded to Aβ1-42 aggregates. For Aβp3-42 aggregates, Rem-CAR elicited the strongest response, followed by Adu-CAR, while E2814-CAR and Don-CAR showed no activation. The activation of Adu-CAR by Aβ1-42 aggregates was recapitulated in primary CD4⁺ T cells, as measured by CD69 expression.
Conclusions: Our findings demonstrate that CARs can detect and discriminate between tau PFFs, Aβ1-42, and Aβp3-42 aggregates. This highlights the potential of repurposing AD antibodies for CAR-based therapies to selectively target tau NFTs and distinct forms of Aβ senile plaques.
Keywords: Aducanumab; Alzheimer’s disease; Amyloid beta; Chimeric antigen receptor; Donanemab; E2814; Lecanemab; Pyroglutamated amyloid beta; Remternetug; Tau.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: JKA, CCW, and SL are inventors in Patent No. WO2024076500A3.
Figures







Update of
-
Chimeric Antigen Receptors Discriminate Between Tau and Distinct Amyloid-Beta Species.bioRxiv [Preprint]. 2025 Feb 5:2025.02.05.636350. doi: 10.1101/2025.02.05.636350. bioRxiv. 2025. Update in: J Transl Med. 2025 May 30;23(1):605. doi: 10.1186/s12967-025-06572-6. PMID: 39974919 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

