Carbon dots from purple sweet potato as a promising anti-inflammatory biomaterial for alleviating the LPS-induced inflammation in macrophages
- PMID: 40448145
- PMCID: PMC12124011
- DOI: 10.1186/s12951-025-03494-9
Carbon dots from purple sweet potato as a promising anti-inflammatory biomaterial for alleviating the LPS-induced inflammation in macrophages
Abstract
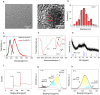
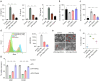
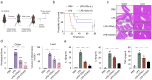
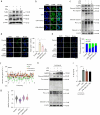
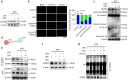
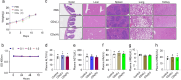
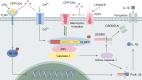
This study synthesizes carbon dots derived from crude extracts of purple sweet potato (CPP-CDs) and evaluates its anti-inflammatory effects in a lipopolysaccharide (LPS) -induced acute inflammation model. Characterization revealed that CPP-CDs possess a uniform spherical structure and excellent photoluminescent properties. In vitro, CPP-CDs significantly inhibited the expression of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α), reduced the accumulation of reactive oxygen species (ROS), suppressed pyroptosis, and facilitated the polarization of macrophages from the M1 phenotype to the M2 phenotype. In vivo, CPP-CDs significantly improved the survival rates of LPS-treated mice, mitigated tissue damage, and suppressed the levels of pro-inflammatory cytokines. Mechanistic studies indicated that CPP-CDs exert anti-inflammatory effects through the inhibition of the TLR4/NF-κB signaling pathway and the modulation of the NLRP3 inflammasome. Additionally, CPP-CDs exhibited excellent biocompatibility, with no significant toxicity observed in mice. This study provides strong evidence supporting the application of CPP-CDs as a novel anti-inflammatory material, highlighting their potential for acute inflammation treatment and expanding the possibilities for the development of carbon-dot-based anti-inflammatory therapies.
Keywords: Anti-inflammatory material; Carbon dots; NLRP3 inflammasome; Purple sweet potato; Reactive oxygen species; TLR4/NF-κB signaling pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The Ethics Committee of the Affiliated Hospital of Nantong University granted approval for this study. All animal procedures were reviewed and authorized by the Animal Care and Use Committee of Nantong University and were carried out in compliance with its guidelines (No: B20240420011). Consent for publication: All authors provided their consent for publication. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Preventive effects of matrine on LPS-induced inflammation in RAW 264.7 cells and intestinal damage in mice through the TLR4/NF-κB/MAPK pathway.Int Immunopharmacol. 2024 Dec 25;143(Pt 2):113432. doi: 10.1016/j.intimp.2024.113432. Epub 2024 Oct 23. Int Immunopharmacol. 2024. PMID: 39447411
-
Protective effects of nordalbergin against LPS-induced endotoxemia through inhibiting MAPK/NF-κB signaling pathway, NLRP3 inflammasome activation, and ROS production.Inflamm Res. 2024 Oct;73(10):1657-1670. doi: 10.1007/s00011-024-01922-4. Epub 2024 Jul 25. Inflamm Res. 2024. PMID: 39052062
-
Purple Sweet Potato Polysaccharide Exerting an Anti-inflammatory Effect via a TLR-Mediated Pathway by Regulating Polarization and Inhibiting the Inflammasome Activation.J Agric Food Chem. 2024 Jan 31;72(4):2165-2177. doi: 10.1021/acs.jafc.3c07511. Epub 2024 Jan 17. J Agric Food Chem. 2024. PMID: 38233194
-
[Daidzein attenuates high glucose-induced inflammatory injury in macrophages by regulating NLRP3 inflammasome signaling pathway].Zhongguo Zhong Yao Za Zhi. 2024 Sep;49(17):4734-4743. doi: 10.19540/j.cnki.cjcmm.20240516.706. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 39307808 Chinese.
-
GEN-27 exhibits anti-inflammatory effects by suppressing the activation of NLRP3 inflammasome and NF-κB pathway.Cell Biol Int. 2019 Oct;43(10):1184-1192. doi: 10.1002/cbin.11101. Epub 2019 Aug 4. Cell Biol Int. 2019. PMID: 30632647
Cited by
-
Amniotic mesenchymal stem cells attenuate diabetic cardiomyopathy by inhibiting pyroptosis via modulation of the TLR4/NF-κb/NLRP3 pathway.Front Cell Dev Biol. 2025 Jul 10;13:1631973. doi: 10.3389/fcell.2025.1631973. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40708699 Free PMC article.
-
pH-modulated synthesis of multicolor carbon dots from Perilla leaves for dual-mode room-temperature fluorescence and phosphorescence applications.Mikrochim Acta. 2025 Aug 14;192(9):589. doi: 10.1007/s00604-025-07460-y. Mikrochim Acta. 2025. PMID: 40804169
References
-
- Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374(6571):1070–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

