GSTM1 suppresses cardiac fibrosis post-myocardial infarction through inhibiting lipid peroxidation and ferroptosis
- PMID: 40448227
- PMCID: PMC12125851
- DOI: 10.1186/s40779-025-00610-6
GSTM1 suppresses cardiac fibrosis post-myocardial infarction through inhibiting lipid peroxidation and ferroptosis
Abstract
Background: Cardiac fibrosis following myocardial infarction (MI) drives adverse ventricular remodeling and heart failure, with cardiac fibroblasts (CFs) playing a central role. GSTM1 is an important member of the glutathione S-transferase (GSTs) family, which plays an important role in maintaining cell homeostasis and detoxification. This study investigated the role and mechanism of GSTM1 in post-MI fibrosis.
Methods: Multi-omics approaches (proteomics/scRNA-seq) identified GSTM1 as a dysregulated target in post-MI fibroblasts. Using a murine coronary ligation model, we assessed GSTM1 dynamics via molecular profiling, such as Western blotting, immunofluorescence, and real-time quantitative polymerase chain reaction. AAV9-mediated cardiac-specific GSTM1 overexpression was achieved through systemic delivery. In vitro studies employed transforming growth factor-β (TGF-β)-stimulated primary fibroblasts with siRNA/plasmid interventions. Mechanistic insights were derived from transcriptomics and lipid peroxidation assays.
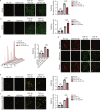
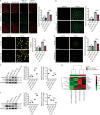
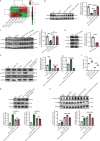
Results: The expression of GSTM1 in mouse CFs after MI was significantly down-regulated at both transcriptional and protein levels. In human dilated cardiomyopathy (DCM) patients with severe heart failure, GSTM1 expression was decreased alongside aggravated fibrosis. Overexpression of GSTM1 in post-MI mice improved cardiac function, while significantly reducing infarct size and fibrosis compared with the control group. In vitro models demonstrated that GSTM1 markedly attenuated collagen secretion and activation of fibroblasts, as well as suppressed their proliferation and migration. Further studies revealed that GSTM1 overexpression significantly inhibited the generation of intracellular and mitochondrial reactive oxygen species (ROS) under pathological conditions, suggesting that GSTM1 exerts an antioxidative stress effect in post-infarction fibroblasts. Further investigation of molecular mechanisms indicated that GSTM1 may suppress the initiation and progression of fibrosis by modulating lipid metabolism and ferroptosis-related pathways. Overexpression of GSTM1 significantly reduced lipid peroxidation and free ferrous iron levels in fibroblasts and mitochondria, markedly decreased ferroptosis-related indicators, and alleviated oxidative lipid levels [such as 12-hydroxyeicosapentaenoic acid (HEPE) and 9-, 10-dihydroxy octadecenoic acid (DHOME)] under fibrotic conditions. GSTM1 enhanced the phosphorylation of STAT3, thereby upregulating the downstream expression of glutathione peroxidase 4 (GPX4), reducing ROS production, and mitigating fibroblast activation and phenotypic transformation by inhibiting lipid peroxidation.
Conclusions: This study identifies GSTM1 as a key inhibitor of fibroblast activation and cardiac fibrosis, highlighting its ability to target ferroptosis through redox regulation. AAV-mediated GSTM1 therapy demonstrates significant therapeutic potential for improving outcomes post-MI.
Keywords: Cardiac fibrosis; Ferroptosis; GSTM1; Glutathione peroxidase 4; Lipid peroxidation; Myocardial infarction (MI); Reactive oxygen species (ROS).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures involving animals have been approved by the Zhejiang University Animal Care and Utilization Committee (2022-113). All procedures involving human samples were approved by the Human Research Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (2014-160). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures





References
-
- Townsend N, Kazakiewicz D, Lucy Wright F, Timmis A, Huculeci R, Torbica A, et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. 2022;19(2):133–43. - PubMed
-
- González A, Schelbert EB, Díez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018;71(15):1696–706. - PubMed
MeSH terms
Substances
Grants and funding
- 82270386 to HM/National Natural Science Foundation of China
- 82070252 to HM/National Natural Science Foundation of China
- 82070251 to MX/National Natural Science Foundation of China
- LR21H020001 to HM/Natural Science Foundation of Zhejiang Province
- 2023RC020 to SY/Medical Science and Technology Project of Zhejiang Province
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

