A Novel Interaction of Staphylococcal Protein A With Human Fibronectin and Its Implications in Host Cell Adhesion
- PMID: 40448417
- PMCID: PMC12125616
- DOI: 10.1096/fj.202500086R
A Novel Interaction of Staphylococcal Protein A With Human Fibronectin and Its Implications in Host Cell Adhesion
Abstract
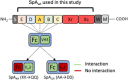
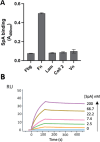
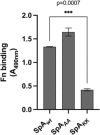
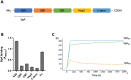
Staphylococcus aureus is the causative agent of serious human health conditions, such as sepsis, endocarditis, and necrotizing pneumonia, as well as less severe clinical manifestations including epithelial and mucosal infections. This pathogen expresses a wide range of surface virulence factors, among which fibronectin-binding proteins play a crucial role in both bacterial adhesion and infection of host cells. Fibronectin is utilized by S. aureus during the early stages of infection to form a protective coating that shields the bacterium from host defenses, facilitating adhesion to the extracellular matrix of host cells and promoting immune evasion and subsequent invasion. S. aureus protein A (SpA) is a key multi-domain cell wall-anchored and secreted molecule that functions to evade the human immune response by non-specifically interacting with Fc and the Fab VH3 domains of immunoglobulins. Other known human ligands of SpA include the von Willebrand factor, the tumor necrosis factor receptor 1, and a platelet surface protein, all of which contribute to immune evasion and pathogenesis. The present study reveals that SpA can also bind human fibronectin with high affinity, adding a new function to this already multifunctional virulence factor. We show that the N-terminus of fibronectin is involved in the interaction and demonstrate by carbene footprinting experiments that the SpA fibronectin binding site spans the interdomain linker region and helix 1 of the domains D, A, B, and C, partially overlapping with the Fc binding site. In the presence of fibronectin, SpA knock-out mutant strains showed reduced adhesion to human endothelial cells compared to wild-type bacteria, suggesting that this interaction may play a significant role in the attachment to host tissues by S. aureus.
Keywords: Staphylococcus aureus; SpA; bacterial adhesion; fibronectin; monoclonal antibody; protein–protein interaction.
© 2025 GSK Vaccines. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
At the time of the study, all authors, excluding G. Pietrocola and
Figures

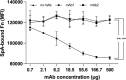
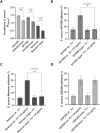
References
-
- Foster T. J., Geoghegan J. A., Ganesh V. K., and Höök M., “Adhesion, Invasion and Evasion: The Many Functions of the Surface Proteins of <styled-content style="fixed-case"> Staphylococcus aureus </styled-content> ,” Nature Reviews. Microbiology 12, no. 1 (2014): 49–62, 10.1038/nrmicro3161. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

