Lenvatinib Plus Pembrolizumab and Chemotherapy Versus Chemotherapy in Advanced Metastatic Gastroesophageal Adenocarcinoma: The Phase III, Randomized LEAP-015 Study
- PMID: 40448579
- PMCID: PMC12288889
- DOI: 10.1200/JCO-25-00748
Lenvatinib Plus Pembrolizumab and Chemotherapy Versus Chemotherapy in Advanced Metastatic Gastroesophageal Adenocarcinoma: The Phase III, Randomized LEAP-015 Study
Abstract
Purpose: The phase III randomized open-label LEAP-015 study (ClinicalTrials.gov identifier: NCT04662710) evaluated first-line lenvatinib plus pembrolizumab and chemotherapy versus chemotherapy for advanced metastatic gastroesophageal adenocarcinoma.
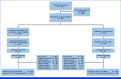
Methods: Eligible participants 18 years and older with untreated human epidermal growth factor receptor 2-negative locally advanced unresectable or metastatic gastroesophageal adenocarcinoma were randomly assigned 1:1 to induction with oral lenvatinib 8 mg once daily plus pembrolizumab 400 mg intravenously once every 6 weeks (×2) and investigators' choice of capecitabine and oxaliplatin once every 3 weeks (×4) or fluorouracil, leucovorin, and oxaliplatin once every 2 weeks (×6) and consolidation with lenvatinib plus pembrolizumab, or chemotherapy. Dual primary end points were progression-free survival (PFS) and overall survival (OS) in participants with PD-L1 combined positive score (CPS) ≥1 and all participants. Secondary end points included objective response rate (ORR) and duration of response.
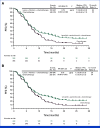
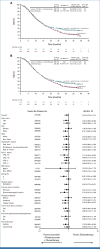
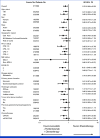
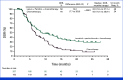
Results: Of 880 participants randomly assigned, 443 received lenvatinib plus pembrolizumab and 437 received chemotherapy. The median follow-ups were 32.2 months (range, 19.0-41.7) in participants with PD-L1 CPS ≥1 and 31.8 months (19.0-41.7) in all participants. At interim analysis, PFS was statistically significant with lenvatinib plus pembrolizumab versus chemotherapy in participants with PD-L1 CPS ≥1 (median, 7.3 v 6.9 months; hazard ratio [HR], 0.75 [95% CI, 0.62 to 0.9]; P = .0012) and all participants (median, 7.2 v 7.0 months; HR, 0.78 [95% CI, 0.66 to 0.92]; P = .0019). The ORR was 59.5% versus 45.4% in participants with PD-L1 CPS ≥1 and 58.0% versus 43.9% in all participants, P < .0001 for both. At final analysis, OS was not statistically significant in participants with PD-L1 CPS ≥1 (median, 12.6 v 12.9 months; HR, 0.84 [95% CI, 0.71 to 1.00]; P = .0244; P value boundary = .0204). Grade ≥3 drug-related adverse event rates were 65% versus 49%.
Conclusion: Lenvatinib plus pembrolizumab and chemotherapy versus chemotherapy provided a statistically significant improvement in PFS in advanced unresectable or metastatic gastroesophageal carcinoma at interim analysis although the clinical significance of this difference seems to be limited. No significant improvement occurred in OS in participants with PD-L1 CPS ≥1.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Rha SY, Oh DY, Yañez P, et al. : Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 24:1181-1195, 2023 - PubMed
-
- Qiu MZ, Oh DY, Kato K, et al. : Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ 385:e078876, 2024 - PubMed
-
- Wilke H, Muro K, Van Cutsem E, et al. : Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol 15:1224-1235, 2014 - PubMed
-
- Ellis LM, Hicklin DJ: VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer 8:579-591, 2008 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

