Listerin Alleviates Alzheimer's Disease through IRE1-mediated Decay of TLR4 mRNA
- PMID: 40448625
- PMCID: PMC12407295
- DOI: 10.1002/advs.202414956
Listerin Alleviates Alzheimer's Disease through IRE1-mediated Decay of TLR4 mRNA
Abstract
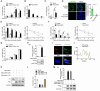
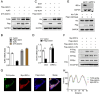
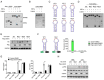
Alzheimer's disease (AD) is the most prevalent neurodegenerative disorder, accounting for ≈60-70% of all dementia cases worldwide. Microglial-mediated brain inflammation is thought to play key roles in AD progression. Clinical evidence and animal models have indicated that the ribosome-associated quality control (RQC) component Listerin is involved in the development of AD. How Listerin regulates the development and progression of AD is unknown. Here, it is demonstrated that Listerin can decrease brain inflammation and alleviate AD-related cognitive impairments. Microglial-specific knockout of Listerin exhibits deteriorative cognitive symptoms based on the extracellular Amyloid-β (Aβ) or Lipopolysaccharide (LPS) injection. Mechanistically, Listerin directly binds to Toll-like receptor 4 (TLR4) mRNA and facilitates the IRE1α-mediated cleavage and degradation of TLR4 mRNA, leading to the alleviation of TLR4-induced brain inflammation. Adenovirus-mediated overexpression of Listerin decelerates the disease progression in the mouse model of Aβ-mediated neurodegeneration. Thus, Listerin is an important suppressor of microglia-induced brain inflammation and may be a potential therapeutic target for AD treatment.
Keywords: Alzheimer's disease; IRE1α‐dependent decay (RIDD); Listerin; TLR4; inflammation.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Dubois B., Villain N., Frisoni G. B., Rabinovici G. D., Sabbagh M., Cappa S., Bejanin A., Bombois S., Epelbaum S., Teichmann M., Habert M. O., Nordberg A., Blennow K., Galasko D., Stern Y., Rowe C. C., Salloway S., Schneider L. S., Cummings J. L., Feldman H. H., Lancet Neurol. 2021, 20, 484. - PMC - PubMed
-
- a) Chhatwal J. P., Schultz S. A., McDade E., Schultz A. P., Liu L., Hanseeuw B. J., Joseph‐Mathurin N., Feldman R., Fitzpatrick C. D., Sparks K. P., Levin J., Berman S. B., Renton A. E., Esposito B. T., Fernandez M. V., Sung Y. J., Lee J. H., Klunk W. E., Hofmann A., Noble J. M., Graff‐Radford N., Mori H., Salloway S. M., Masters C. L., Martins R., Karch C. M., Xiong C., Cruchaga C., Perrin R. J., Gordon B. A., et al., Lancet Neurol. 2022, 21, 140; - PMC - PubMed
- b) Ossenkoppele R., van der Kant R., Hansson O., Lancet Neurol. 2022, 21, 726; - PubMed
- c) Tijms B. M., Gobom J., Reus L., Jansen I., Hong S., Dobricic V., Kilpert F., Ten Kate M., Barkhof F., Tsolaki M., Verhey F. R. J., Popp J., Martinez‐Lage P., Vandenberghe R., Lleó A., Molinuevo J. L., Engelborghs S., Bertram L., Lovestone S., Streffer J., Vos S., Bos I., Blennow K., Scheltens P., Teunissen C. E., Zetterberg H., Visser P. J., Brain 2020, 143, 3776. - PMC - PubMed
-
- a) Heneka M. T., Carson M. J., El Khoury J., Landreth G. E., Brosseron F., Feinstein D. L., Jacobs A. H., Wyss‐Coray T., Vitorica J., Ransohoff R. M., Herrup K., Frautschy S. A., Finsen B., Brown G. C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G. C., Town T., Morgan D., Shinohara M. L., Perry V. H., Holmes C., Bazan N. G., Brooks D. J., Hunot S., Joseph B., Deigendesch N., et al., Lancet Neurol. 2015, 14, 388; - PMC - PubMed
- b) De Strooper B., Karran E., Cell 2016, 164, 603. - PubMed
-
- Heneka M. T., van der Flier W. M., Jessen F., Hoozemanns J., Thal D. R., Boche D., Brosseron F., Teunissen C., Zetterberg H., Jacobs A. H., Edison P., Ramirez A., Cruchaga C., Lambert J. C., Laza A. R., Sanchez‐Mut J. V., Fischer A., Castro‐Gomez S., Stein T. D., Kleineidam L., Wagner M., Neher J. J., Cunningham C., Singhrao S. K., Prinz M., Glass C. K., Schlachetzki J. C. M., Butovsky O., Kleemann K., De Jaeger P. L., et al., Nat. Rev. Immunol. 2025, 25, 321. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
