The impact of metformin on placental ageing in humans and mice
- PMID: 40448705
- PMCID: PMC12148204
- DOI: 10.1113/JP288710
The impact of metformin on placental ageing in humans and mice
Abstract
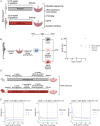
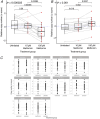
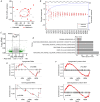
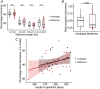
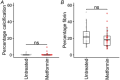
Placental ageing refers to the physiological accumulation of a senescent phenotype over a healthy pregnancy. In pregnancies affected by complications such as pre-eclampsia and fetal growth restriction, placental ageing is notably accelerated and observed at an earlier gestational age. Metformin is used during pregnancy for an increasing variety of indications, including treatment of gestational diabetes, and may have a role in slowing cellular ageing. It is therefore essential to understand the potential impact of metformin on placental ageing. Placental samples (n = 105) were obtained from women with body mass index ≥30 kg/m2 and who were randomized to treatment with metformin or placebo during pregnancy. Ageing was assessed by measuring telomere length, histological examination, and using array-based technologies to investigate gene expression and methylation. Results were validated using isolated human trophoblasts treated in vitro with metformin, and in a complementary mouse model. There were no differences between metformin-exposed and control placentas in terms of telomere length, fibrosis or calcification. There were no differences in placental gene expression or methylation patterns by metformin status. In our mouse model, no genes classically associated with cellular ageing were differentially expressed and no senescence pathway showed evidence of enrichment with metformin treatment. There was no evidence that metformin either slows or accelerates placental ageing pathways in the complementary models that we investigated. Our findings are reassuring with regard to the safety of metformin used to treat gestational diabetes, but do not support a role for metformin in the prevention of adverse pregnancy outcomes in non-diabetic women. KEY POINTS: Accelerated placental ageing, where the senescent phenotype that normally accumulates over a healthy pregnancy is observed at a premature gestational age, is associated with adverse pregnancy outcomes. Metformin has been proposed as an anti-ageing drug elsewhere. Therefore, metformin could alter the trajectory of placental ageing and prevent associated pregnancy complications. The present study incorporated human data from a randomized clinical trial and complementary models. Metformin did not impact methylation-predicted gestational age, telomere length, gene expression or histological ageing in human placentas treated in vivo, isolated trophoblasts treated in vitro or mouse models. Metformin neither decelerated nor accelerated placental ageing, thereby supporting its continued use in the obstetric setting, for instance in the treatment of gestational diabetes. Metformin cannot be recommended to prevent adverse pregnancy outcomes because we found no evidence suggesting it decelerates placental ageing. Further research is warranted to find drug therapies for this purpose.
Keywords: fetus; gestational diabetes; metformin; placenta; pregnancy; trophoblast.
© 2025 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
None declared.
Figures

References
-
- Aiken, C. E. , Tarry‐Adkins, J. L. , Spiroski, A. M. , Nuzzo, A. M. , Ashmore, T. J. , Rolfo, A. , Sutherland, M. J. , Camm, E. J. , Giussani, D. A. , & Ozanne, S. E. (2019). Chronic gestational hypoxia accelerates ovarian aging and lowers ovarian reserve in next‐generation adult rats. Federation of American Societies for Experimental Biology Journal, 33(6), 7758–7766. - PMC - PubMed
-
- Akram, K. M. , Kulkarni, N. S. , Brook, A. , Wyles, M. D. , & Anumba, D. O. C. (2022). Transcriptomic analysis of the human placenta reveals trophoblast dysfunction and augmented wnt signalling associated with spontaneous preterm birth. Frontiers in Cell and Developmental Biology, 10, 987740. - PMC - PubMed
-
- Catalano, P. M. , & Ehrenberg, H. M. (2006). The short‐ and long‐term implications of maternal obesity on the mother and her offspring. British Journal of Obstetrics and Gynaecology, 113(10), 1126–1133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RG/17/12/33167/BHF_/British Heart Foundation/United Kingdom
- MC_UU_00039/MRC_/Medical Research Council/United Kingdom
- 208363/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- 146281/NIHR Cambridge Biomedical Research Centre
- MR/R014167/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00014/4/MRC_/Medical Research Council/United Kingdom
- RE/18/5/34216/BHF_/British Heart Foundation/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/T016701/1/MRC_/Medical Research Council/United Kingdom
- PG/20/11/34957/BHF_/British Heart Foundation/United Kingdom
- 226800/Z/22/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
