Clinical Management and Outcomes of Immune-Related Adverse Events During Treatment with Immune Checkpoint Inhibitor Therapies in Melanoma and Renal Cell Carcinoma: A UK Real-World Evidence Study
- PMID: 40448748
- PMCID: PMC12379197
- DOI: 10.1007/s40487-025-00349-z
Clinical Management and Outcomes of Immune-Related Adverse Events During Treatment with Immune Checkpoint Inhibitor Therapies in Melanoma and Renal Cell Carcinoma: A UK Real-World Evidence Study
Abstract
Introduction: Immune checkpoint inhibitor (ICI) therapy, although effective in treating patients with a variety of advanced malignancies, can result in potentially severe or even fatal immune-related adverse events (irAEs). This study aimed to generate real-world evidence on irAE characteristics, clinical management and clinical outcomes among patients with advanced (unresectable or metastatic) malignant melanoma (a/mMM) or advanced renal cell carcinoma (aRCC) treated with nivolumab (NIVO) and/or ipilimumab (IPI) in the UK.
Methods: This was a multi-centre, retrospective cohort study of adult patients with a/mMM or aRCC, who received NIVO and/or IPI at one of five specialist treatment centres in the UK between 1 January 2016 and 31 March 2020. The incidence and grading of irAEs were described, as well as time to irAE onset, the management of irAEs and overall survival (OS).
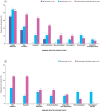
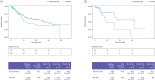
Results: In total, 199 patients were included in the study: 162 with a/mMM and 37 with aRCC. The majority of patients in both a/mMM (75.3%) and aRCC (62.2%) cohorts reported any irAE, while 45.9% and 30.4% in the a/mMM and aRCC cohorts reported grade 3 or 4 irAEs, respectively. Colitis/diarrhoea, skin reactions and hepatitis were most frequently reported, and the predominant treatment prescribed for any irAE was corticosteroids only. Analysis indicated a positive association between the development of an irAE and longer OS.
Conclusions: Findings from this study support current literature, provide further insights into the characteristics and clinical management of irAEs and support an association between the development of an irAE and improved OS in these patient groups.
Keywords: Advanced or metastatic malignant melanoma (a/mMM); Advanced renal cell carcinoma (aRCC); Immune checkpoint inhibitors (ICI); Immune-related adverse events (irAEs); irAE management.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: NHS trusts employing Anna Olsson-Brown, Ankit Jain, Ricky Frazer, David Farrugia and Judith Carser received fees from Bristol Myers Squibb for data collection in relation to this study. In addition, Anna Olsson-Brown has previously received honoraria and/or congress support from Bristol Myers Squibb, MSD, Astra Zeneca, Ipsen, Roche, Regeneron, GSK, Eisai and Chugai; Ankit Jain has received honoraria and/or congress support from MSD, Merck, Takeda, Pfizer, Astra Zeneca and Roche; Ricky Frazer has received speaking and/or advisory fees from Bristol Myers Squibb, Eisai, Ewopharma, Ipsen, Merck, MSD, Novartis, Pfizer, Pierre Fabre, Recordati, Roche, Sanofi and Servier; David Farrugia is employed by Merck Sharpe and Dohme (since May 2024) and Judith Carser declares no additional competing interests. John Houghton and Ruth Lewis are employees of Health Economics and Outcomes Research Ltd, which received fees from Bristol Myers Squibb in relation to this study. Simon D’Mello and Gabrielle Emanuel are employees of Bristol Myers Squibb. Ethical Approval: Approval was obtained from the Health Research Authority (HRA) and Health and Care Research Wales (HCRW) (IRAS Project ID: 284489), with minor amendments submitted and approved on 19 October 2021 and 14 December 2021, respectively. This study did not require research ethics committee (REC) review or informed patient consent because it used previously collected data that was not identifiable outside of the direct care team (Reference: 21/PR/0474).
Figures

References
-
- European Medicines Agency. OPDIVO Summary of Product Characteristics. 2024; Available from: https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-p....
-
- European Medicines Agency. YERVOY summary of product characteristics. 2023 18 February 2024]; Available from: https://www.ema.europa.eu/en/documents/product-information/yervoy-epar-p....
-
- National Institute for Health Care Excellence. TA400 Nivolumab in combination with ipilimumab for treating advanced melanoma. 2016; Available from: https://www.nice.org.uk/guidance/ta400.
-
- National Institute for Health Care Excellence. TA581 Nivolumab with ipilimumab for untreated advanced renal cell carcinoma. 2019; Available from: https://www.nice.org.uk/guidance/ta581. - PubMed
LinkOut - more resources
Full Text Sources

