Focal High-Grade Areas with a Tumor-in-Tumor Pattern: Another Feature of Pediatric DICER1-Associated Thyroid Carcinoma?
- PMID: 40448797
- PMCID: PMC12126348
- DOI: 10.1007/s12022-025-09863-2
Focal High-Grade Areas with a Tumor-in-Tumor Pattern: Another Feature of Pediatric DICER1-Associated Thyroid Carcinoma?
Abstract
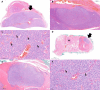
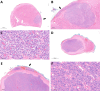
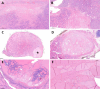
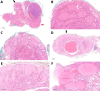
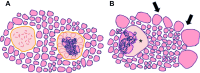
In the thyroid gland, during childhood or adolescence, DICER1-driven tumors include differentiated follicular thyroid carcinoma and, more rarely, poorly differentiated carcinoma. Herein, we describe the features of DICER1-associated thyroid carcinoma with the presence of high-grade areas within a differentiated tumor in four patients (median age 12.5 years, range 6-15 years), three of them carrying germline pathogenic variants of DICER1. A new tumor-in-tumor pattern characterized by intratumoral nodules with a higher histological grade (increased mitotic activity/Ki-67 and solid/trabecular/insular and/or microfollicular architecture) was detected in these DICER1-associated tumors. In two patients, the high-grade component also demonstrated the presence of CHEK2 p.(Tyr390Cys) likely pathogenic variants, suggesting a role for this gene and more generally for the ATM-CHECK2-TP53 pathway as a mechanism of malignant progression of DICER1-associated thyroid carcinomas. One of these two patients presented lymph node recurrence 8 months after surgery. An immunohistochemical study was also performed to explore the possible contribution of anti-DICER1 antibodies as well as thyroglobulin, Ki-67, p53, and PRAME in characterizing these tumors. DICER1 proved to be strongly expressed in mutated tumors compared to a control cohort (p < 0.001), deserving further validation to define its possible diagnostic role. Finally, well-demarcated ischemic-like areas with ghost cells embedded in a thick hyaline stroma (atrophic changes) were found within four tumors, whereas bunches of ectatic macrofollicles lined by flattened epithelium (involutional changes) were only detected in the background thyroid parenchyma of patients with germline DICER1 variants. These morphological features may alert pathologists to suspect a somatic and/or germline DICER1 alteration.
Keywords: CHEK2; DICER1; High-grade; Immunohistochemistry; Pediatric thyroid cancer; Poorly differentiated thyroid carcinoma; Thyroid; Tumor-in-tumor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. As per Italian law (resolution March 1, 2012, Gazzetta Ufficiale n.72 of March 26, 2012), due to the retrospective nature of the study, utilization of anonymous data, and its lack of impact on patient management, Ethical Committee approval was waived. The study procedures adhered to ethical standards set by the institutional and national research committee, aligning with the principles outlined in the 1964 Helsinki declaration and subsequent amendments or equivalent ethical norms. This study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Competing interests: The authors declare no competing interests.
Figures





References
-
- WHO Classification of Tumours Editorial Board. Endocrine and neuroendocrine tumours. Lyon (France): International Agency for Research on Cancer; 2022. (WHO classification of tumours series, 5th ed.; vol. 10). Available from: https://tumourclassification.iarc.who.int/chapters/53.
-
- Nosé V, Gill A, Teijeiro JMC, Perren A, Erickson L. Overview of the 2022 WHO Classification of Familial Endocrine Tumor Syndromes. Endocr Pathol. 2022 Mar;33(1):197-227. 10.1007/s12022-022-09705-5. - PubMed
-
- Anglesio MS, Wang Y, Yang W, Senz J, Wan A, Heravi-Moussavi A, Salamanca C, Maines-Bandiera S, Huntsman DG, Morin GB. Cancer-associated somatic DICER1 hotspot mutations cause defective miRNA processing and reverse-strand expression bias to predominantly mature 3p strands through loss of 5p strand cleavage. J Pathol. 2013 Feb;229(3):400-9. 10.1002/path.4135. - PubMed
-
- Foulkes WD, Priest JR, Duchaine TF. DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer. 2014 Oct;14(10):662-72. 10.1038/nrc3802. - PubMed
-
- Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M, Tallini G, Mete O. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr Pathol. 2022 Mar;33(1):27-63. 10.1007/s12022-022-09707-3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

