DLK1-expressing neural progenitor cells promote tissue repair and functional recovery after cervical spinal cord injury
- PMID: 40448964
- PMCID: PMC12126085
- DOI: 10.1093/stcltm/szaf014
DLK1-expressing neural progenitor cells promote tissue repair and functional recovery after cervical spinal cord injury
Abstract
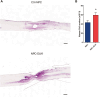
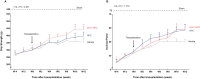
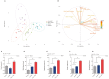
Spinal cord injury (SCI) elicits a hostile microenvironment characterized by inflammation, gliosis, and disrupted signaling pathways that collectively impede neural repair. Neural progenitor cells (NPCs) represent a promising regenerative approach, yet their survival and differentiation are often compromised in this setting. Here, we investigated whether engineering NPCs to overexpress the Notch pathway modulator Delta-like non-canonical Notch ligand 1 (DLK1) could overcome these limitations and improve functional outcomes after cervical SCI in rats. NPCs were engineered to express DLK1 under a Pax6 promoter-driven expression system, ensuring elevated DLK1 levels during the progenitor state. Following transplantation of DLK1-overexpressing NPCs or control NPCs, we assessed graft survival, lineage differentiation, behavioral performance, and electrophysiological integration over 12 weeks. DLK1-expressing NPCs exhibited significantly greater retention in the injured spinal cord and showed enhanced neuronal differentiation alongside reduced astrocytic commitment compared to controls. Behavioral tests-including forelimb grip strength and CatWalk gait assessments-demonstrated that DLK1-modified NPCs conferred robust improvements in forelimb motor coordination and overall locomotion. Concordantly, electrophysiological recordings revealed increased motor-evoked potential amplitudes and area-under-the-curve values in animals receiving DLK1-transduced NPC grafts, indicative of strengthened synaptic integration within the host motor circuitry.
Keywords: DLK1; axons; delta-like non-canonical Notch ligand 1; functional recovery; neural progenitor cells; spinal cord injury; tissue repair.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures



References
-
- Hejrati N, Fehlings MG.. A review of emerging neuroprotective and neuroregenerative therapies in traumatic spinal cord injury. Curr Opin Pharmacol. 2021;60:331-340. https://doi.org/ 10.1016/j.coph.2021.08.009 - DOI - PubMed
-
- Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. https://doi.org/ 10.1038/nrdp.2017.18 - DOI - PubMed
-
- Hosseini SM, Borys B, Karimi-Abdolrezaee S.. Neural stem cell therapies for spinal cord injury repair: an update on recent preclinical and clinical advances. Brain. 2024;147:766-793. https://doi.org/ 10.1093/brain/awad392 - DOI - PubMed
-
- Nishimura S, Yasuda A, Iwai H, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. https://doi.org/ 10.1186/1756-6606-6-3 - DOI - PMC - PubMed
-
- Peng Z, Li X, Fu M, et al. Inhibition of Notch1 signaling promotes neuronal differentiation and improves functional recovery in spinal cord injury through suppressing the activation of Ras homolog family member A. J Neurochem. 2019;150:709-722. https://doi.org/ 10.1111/jnc.14833 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

