Histone lactylation-induced premature senescence contributes to 1-nitropyrene-Induced chronic obstructive pulmonary disease
- PMID: 40449094
- PMCID: PMC12164224
- DOI: 10.1016/j.redox.2025.103703
Histone lactylation-induced premature senescence contributes to 1-nitropyrene-Induced chronic obstructive pulmonary disease
Abstract
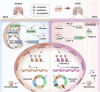
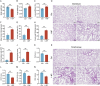
Our previous study revealed that mice exposed to 1-nitropyrene (1-NP) develop pulmonary fibrosis and senescent alveolar cells. However, the impacts of chronic 1-NP on chronic obstructive pulmonary disease (COPD) and the underlying mechanism are unclear. Our research suggested that chronic 1-NP evoked alveolar structure damage, inflammatory cell infiltration, and pulmonary function decline in mice. Moreover, 1-NP increased p53 and p21 expression, the number of β-galactosidase-positive cells, and cell cycle arrest in mouse lungs and MLE-12 cells. Moreover, 1-NP promoted glycolysis and upregulated lactic dehydrogenase A (LDHA) and lactate production in mouse lungs and MLE-12 cells. Elevated glycolysis provoked histone lactylation, but not histone acetylation in pulmonary epithelial cells. Mechanistically, histone H3 lysine 14 lactylation (H3K14la) was upregulated in pulmonary epithelial cells. P53 knockdown mitigated 1-NP-induced cell cycle arrest and senescence in MLE-12 cells. CUT&Tag and ChIP-qPCR experiments confirmed that increased H3K14la directly upregulated p53 transcription in pulmonary epithelial cells. As expected, LDHA knockdown alleviated 1-NP-triggered cell cycle arrest and senescence in MLE-12 cells. In addition, supplementation with oxamate, an inhibitor of LDH, attenuated 1-NP-incurred premature senescence and the COPD-like phenotype in mice. These data revealed for the first time that histone lactylation-induced the increase in p53 transcription contributes to pulmonary epithelial cell senescence during 1-NP-induced COPD progression. Our results provide a basis for repressing lactate production as a promising therapeutic strategy for COPD.
Keywords: 1-Nitropyrene; COPD; Cellular senescence; Histone lactylation; P53.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










References
-
- Bandowe B., Meusel H. Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment - a review. Sci. Total Environ. 2017;581–582:237–257. - PubMed
-
- Gao Y., Yang L., Chen J., Li Y., Jiang P., Zhang J., et al. Nitro and oxy-PAHs bounded in PM2.5 and PM1.0 under different weather conditions at Mount Tai in Eastern China: sources, long-distance transport, and cancer risk assessment. Sci. Total Environ. 2018;622–623:1400–1407. - PubMed
-
- Deng K., Chan W. Development of a QuEChERS-based method for determination of carcinogenic 2-nitrofluorene and 1-nitropyrene in rice grains and vegetables: a comparative study with benzo[a]pyrene. J. Agric. Food Chem. 2017;65(9):1992–1999. - PubMed
-
- Feng S.J., Shen X.B., Hao X.W., Cao X.Y., Li X., Yao X.L., et al. Polycyclic and nitro-polycyclic aromatic hydrocarbon pollution characteristics and carcinogenic risk assessment of indoor kitchen air during cooking periods in rural households in North China. Environ. Sci. Pollut. Res. Int. 2021;28(9):11498–11508. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

