Endolysosomal processing of neuron-derived signaling lipids regulates autophagy and lipid droplet degradation in astrocytes
- PMID: 40450042
- PMCID: PMC12126518
- DOI: 10.1038/s41467-025-60402-3
Endolysosomal processing of neuron-derived signaling lipids regulates autophagy and lipid droplet degradation in astrocytes
Abstract
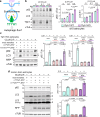
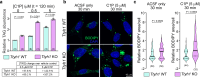
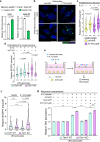
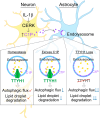
Dynamic regulation of metabolic activities in astrocytes is critical to meeting the demands of other brain cells. During neuronal stress, lipids are transferred from neurons to astrocytes, where they are stored in lipid droplets (LDs). However, it is not clear whether and how neuron-derived lipids trigger metabolic adaptation in astrocytes. Here, we uncover an endolysosomal function that mediates neuron-astrocyte transcellular lipid signaling. We identify Tweety homolog 1 (TTYH1) as an astrocyte-enriched endolysosomal protein that facilitates autophagic flux and LD degradation. Astrocyte-specific deletion of mouse Ttyh1 and loss of its Drosophila ortholog lead to brain accumulation of neutral lipids. Computational and experimental evidence suggests that TTYH1 mediates endolysosomal clearance of ceramide 1-phosphate (C1P), a sphingolipid that dampens autophagic flux and LD breakdown in mouse and human astrocytes. Furthermore, neuronal C1P secretion induced by inflammatory cytokine interleukin-1β causes TTYH1-dependent autophagic flux and LD adaptations in astrocytes. These findings reveal a neuron-initiated signaling paradigm that culminates in the regulation of catabolic activities in astrocytes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests
Figures




References
-
- Bonvento, G. & Bolanos, J. P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab.33, 1546–1564 (2021). - PubMed
-
- Mishra, S. K. & Tiwari, S. P. Bioenergetics of axon integrity and its regulation by oligodendrocytes and schwann cells. Mol. Neurobiol.61, 5928–5934 (2024). - PubMed
-
- Pellerin, L. et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia55, 1251–1262 (2007). - PubMed
MeSH terms
Substances
Grants and funding
- R01AG081379/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P40 OD018537/OD/NIH HHS/United States
- R03AG063251/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R03TR004191/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AG081379/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

