Anlotinib plus toripalimab as a first-line treatment in patients with advanced gastric cancer and performance status 2: the phase II APICAL-GC trial
- PMID: 40450051
- PMCID: PMC12126510
- DOI: 10.1038/s41467-025-60317-z
Anlotinib plus toripalimab as a first-line treatment in patients with advanced gastric cancer and performance status 2: the phase II APICAL-GC trial
Abstract
Evidence-guided regimens for advanced gastric cancer (AGC) in patients with performance status 2 (PS 2) are limited. Here, we proposed a structured therapeutic framework termed "performance status-matched strategy", and further conducted the APICAL-GC trial (NCT04278222). This open-label, single-arm phase II study evaluated the efficacy and safety of anlotinib combined with toripalimab among 24 treatment-naïve AGC patients with PS 2. The primary outcome was the objective response rate (ORR), with secondary endpoints including disease control rate (DCR), duration of response (DoR), progression-free survival (PFS), overall survival (OS), and safety profile. This trial met its prespecified endpoints, demonstrating an ORR of 58.3% (95%CI 36.6-77.9) with a DoR of 12.1 months (range: 1.43-48.5), and a DCR of 95.8% (95%CI 78.9-99.9). Median PFS reached 7.33 months (95%CI 3.83-17.1), while median OS was 15.9 months (95%CI 7.73-23.2). Treatment-related adverse events (TRAEs) of any grade occurred in 21 patients (87.5%), with grade-3 TRAEs observed in 7 patients (29.2%). No grade-4/5 TRAEs were reported. These findings provide a rationale for anlotinib plus toripalimab as a promising chemotherapy-free option for the first-line treatment of AGC patients with PS 2 under the performance status-matched strategy, showing comparable anticancer activity and a lower occurrence rate of TRAEs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: The study was approved by the institutional review board of the ethics committee of Shanghai Changzheng Hospital. Informed consent: Written informed consent was obtained from all patients before any study-related procedures performed. Both the collection of all data in this study and the adoption of the materials comply with the Declaration of Helsinki and the relevant provisions of the guidelines.
Figures



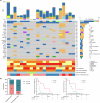
References
-
- Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin.74, 12–49 (2024). - PubMed
-
- Talarico, L., Chen, G. & Pazdur, R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J. Clin. Oncol.22, 4626–4631 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

