Preparation, characterization, and application of a valine-malonic acid based-DES as a potent catalyst for the green synthesis of thiazolopyrimidines
- PMID: 40450071
- PMCID: PMC12126544
- DOI: 10.1038/s41598-025-03630-3
Preparation, characterization, and application of a valine-malonic acid based-DES as a potent catalyst for the green synthesis of thiazolopyrimidines
Abstract
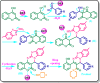
Based on the eutectic point phase diagram, a novel natural deep eutectic solvent (VAL/MAL-DES) was prepared by a mixture of one mole of valine (VAL) and one mole of malonic acid (MAL), and characterized by the eutectic points, FT-IR, 1HNMR, TGA/DTA and densitometer techniques. Then, it was used as a novel and capable catalyst for the green synthesis of thiazolo[3,2-a]pyrimidines via a one-pot four-component condensation reaction of 4-hydroxycoumarin, 2-amino-6-methylbenzothiazole, morpholine, and aldehydes at 80 °C with high yield and short reaction time.
Keywords: Homogeneous catalysis; Malonic acid; Multicomponent reactions; Natural deep eutectic solvent; Valine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Buzatu, A. R., Todea, A., Peter, F. & Boeriu, C. G. The role of reactive natural deep eutectic solvents in sustainable biocatalysis. ChemCatChem13, 202301597 (2024).
-
- Santana-Mayor, Á., Rodríguez-Ramos, R., Herrera-Herrera, A. V., Socas-Rodríguez, B. & Rodríguez-Delgado, M. Á. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC-Trends Anal. Chem.134, 116108 (2021).
-
- Abbott, A. P., Boothby, D., Capper, G., Davies, D. L. & Rasheed, R. K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc.126, 9142–9147 (2004). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

