Modulatory effect of metformin and its transporters on immune infiltration in tumor microenvironment: a bioinformatic study with experimental validation
- PMID: 40450131
- PMCID: PMC12126455
- DOI: 10.1007/s12672-025-02766-y
Modulatory effect of metformin and its transporters on immune infiltration in tumor microenvironment: a bioinformatic study with experimental validation
Abstract
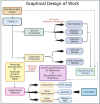
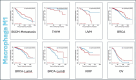
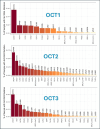
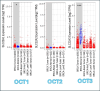
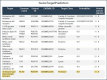
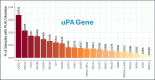
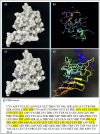
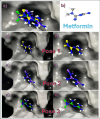
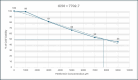
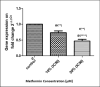
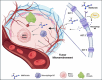
Metformin is a traditional antidiabetic drug for type 2 diabetes mellitus. However, it showed antitumor activity in many types of tumors, and it also has an influence on tumor metastasis in several types of tumors. It is transported through organic cationic transporters (OCTs), OCT1, OCT2, and OCT3, into the cells or into tumor microenvironment (TME). The complex interaction of metformin and its transporters on immune infiltration in TME of different types of tumors of The Cancer Genomic Atlas (TCGA) is not yet studied. The objective of this study is to identify the most suitable therapeutic target of tumors and immune infiltrates for metformin and its transporters in the TME. TIMER2.0, a bioinformatic tool, and other computational analysis were used to investigate this complex interaction; moreover, the identification of metformin target protein in TME is also investigated. The results revealed that the most suitable therapeutic target for metformin and OCTs among 32 types of TCGA data tumor types is Breast Invasive carcinoma (BRCA), and the most relevant immune infiltrate among 14 types of immune infiltrates that yields better prognosis and better therapeutical effect in TME is Macrophage M1. Furthermore, metformin showed a cytotoxic effect and an inhibitory effect on Urokinase Plasminogen Activator (uPA) gene expression in a concentration dependent fashion in MDA-MB-231 breast cancer cell line. This may suggest that metformin is a promising antitumor drug, stimulant for natural antitumor immune infiltrates, and inhibitor for metastasis in breast cancer.
Keywords: Immune infiltrates; Metformin; Organic cationic transporter; TIMER2.0; Tumor microenvironment; Urokinase plasminogen activator.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval was waived by the local Ethics Committee of Al-Azhar University in view of the retrospective nature of the study and all the procedures being performed were part of the routine care. Competing interests: The authors declare no competing interests.
Figures


References
-
- Ozer H, et al. 2000 Update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. American Society of Clinical Oncology Growth Factors Expert Panel. J Clin Oncol Off J Am Soc Clin Oncol. 2000;18(20):3558–85. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
