Saliva-based lacosamide monitoring paves the way toward personalized epilepsy pharmacotherapy
- PMID: 40450138
- PMCID: PMC12126552
- DOI: 10.1038/s41598-025-04044-x
Saliva-based lacosamide monitoring paves the way toward personalized epilepsy pharmacotherapy
Abstract
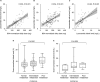
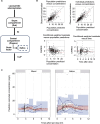
Saliva, known for better patient compliance and simpler collection, is ideal for monitoring antiseizure medication (ASM) levels. This study aimed to validate saliva for measuring lacosamide, develop a pharmacokinetic (PK) model, and determine the optimal saliva concentration for seizure control in epilepsy patients. In our prospective study at Seoul National University Hospital from August 2021 to November 2022, we enrolled lacosamide-prescribed epilepsy patients, collecting their saliva and blood samples. We developed a population PK model with nonlinear mixed-effects modeling, incorporating a saliva compartment and plasma-to-saliva distribution scaling factor. The model, factoring in CYP2C19 genotypes, demographics, and concurrent ASM use, estimated optimal saliva lacosamide concentration cutoffs for well-controlled seizures in high seizure burden patients. These values were validated through a two-year longitudinal analysis. In our study, 123 epilepsy patients prescribed lacosamide were finally analyzed. We identified 74 matched pairs of blood and saliva samples, finding a linear relationship between their lacosamide concentrations (R = 0.62, P < 0.001). Using our PK model, we estimated individual peak (Cmax) and trough concentrations in saliva and blood based on dosage, determining optimal saliva cutoffs for well-controlled seizure status in lacosamide: 15.94 mg/L for Cmax and 9.056 mg/L for trough, with 72.7% sensitivity and 88.2% specificity. Longitudinal analysis showed well-controlled seizure status achievement aligning with times when estimated Cmax and trough surpassed these cutoffs. Our research presents the potential and validity of using saliva concentration as an alternative to blood concentration for lacosamide TDM, advancing personalized pharmacotherapy in epilepsy treatment.
Keywords: Lacosamide; Lacosamide pharmacokinetics; Personalized medicine; Saliva-based TDM.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study was approved by the Seoul National University Hospital Institutional Review Board (IRB No. 2104-146-1213) and written informed consent was obtained from all patients and/or legal guardians. All co-authors have reviewed and approved the contents of the manuscript, and the Scientific Reports requirements for authorship have been met. We confirm that we have read the journal’s position on issues related to ethical publication and affirm that this report is consistent with those guidelines. We certify that the submission (aside from an abstract) is not under review by any other publication. No previous report overlaps with the current work. We have no conflicts of interest to declare.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

