Study on characteristics of gut flora composition of pregnant women with preeclampsia
- PMID: 40450263
- PMCID: PMC12125917
- DOI: 10.1186/s12884-025-07760-4
Study on characteristics of gut flora composition of pregnant women with preeclampsia
Abstract
Objectives: To explore the relationship between preeclampsia and the changes of the intestinal microflora.
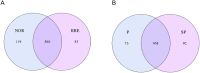
Methods: Illumina HiSeq sequencing platform was used to sequencing the 16S rRNA of bacteria in stool samples from 54 pregnant women. The experimental group included 27 women with preeclampsia (PRE), consisting of 13 women with severe preeclampsia (SP) and 14 women with non-severe preeclampsia (P). The control group comprised 27 healthy pregnant women (NOR).
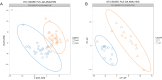
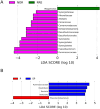
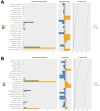
Results: The bacterial Alpha diversity of the PRE group was higher than that of the normal group, but P>0.05. The dominant flora in the four groups were Bacteroidetes, Firmicutes, and Proteobacteria. The abundance of Synergistetes in the PRE group was significantly lower than that in normal group and Bacteroidetes in the P group was significantly higher than that in SP group, and Firmicutes in the P group was significantly lower than that in the SP group(P < 0.05). LEfSe analyses showed, there were 9 flora differences between PRE group and NOR group, between P and SP groups that only Ruminococcaceae showed a difference. Differential species screening showed, there are 3 flora were significantly different between the PRE vs. NOR groups (P < 0.05). There are 4 flora were significantly different between the P vs. SP groups (P < 0.05). Functional difference analysis showed significant differences in 8 signaling pathways between the NOR group and the PRE group and 11 signaling pathways between P group and SP group.
Conclusion: There are significant changes in intestinal flora between preeclampsia patients and healthy controls, which may be involved in the regulation of the occurrence and development of preeclampsia.
Keywords: Biomarker; Intestinal flora; Metabolic diseases; Preeclampsia; Pregnancy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The protocol of the study was approved by the ethical committee of third affiliated hospital of Zhengzhou University(2018 Medical Ethics Review No. 14), China. All participants were told about the purpose of the study and informed written consent was taken from all study subjects. This study strictly adhered to the ethical principles outlined in the Declaration of Helsinki (World Medical Association, 2013 revision). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

