Resveratrol reverses cassava (Manihot esculenta Crantz) juice-induced motor impairment in the Wistar rat
- PMID: 40450293
- PMCID: PMC12125863
- DOI: 10.1186/s12906-025-04934-7
Resveratrol reverses cassava (Manihot esculenta Crantz) juice-induced motor impairment in the Wistar rat
Abstract
Background: Long-term cassava (Manihot esculenta Crantz) intake has been associated with the development of neurological diseases characterized by motor impairment in humans and experimental animals. Thus, there is a need to identify the therapeutic effects of molecules to ameliorate said alterations, such as resveratrol, which was explored in the present study. Therefore, we evaluate whether the behavioral alterations associated with the chronic intake of cassava juice could be reversed with resveratrol.
Methods: Adult male rats were randomly assigned to four independent groups (n = 8): vehicle (purified water), cassava (28.56 mg/kg), resveratrol (10.70 mg/kg), and a combination of treatments (cassava plus resveratrol). Vehicle and cassava juices were administered from days 1 to 28, followed by vehicle or resveratrol from days 29 to 56. The effects of the treatments were evaluated on days 28 and 56 in the open field test, rotarod, and swimming test, compared with the baseline.
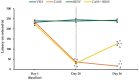
Results: Cassava juice increased crossing, rearing, and grooming in the open field, produced a short latency to fall from the rotarod, and increased the spin behavior and the total time of immobility in the swimming test. These effects were reversed by resveratrol.
Conclusions: Resveratrol could be considered in the development of therapeutic strategies for neurological disorders associated with cassava consumption.
Clinical trial number: Not applicable.
Keywords: Antioxidant; Cassava juice; Motor impairment; Neurologic disease; Resveratrol.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Experimental manipulations were carried out according to international ethical guidelines based on the Guide for the Care and Use of Laboratory Animals published by the National Research Council [98], as well as national regulations based on NOM-062-ZOO1999 [38]. Moreover, the recommendations proposed by Russell’s three Rs (reduce, replace, and refine), focused on experimental animal research, were considered [99]. The protocol was approved by the Internal Committee for the Care and Use of Laboratory Animals of the Institute of Health Sciences of the Universidad Veracruzana, whose registration number is CICUAL-ICS 2018-0002B dated 18 February 2019. All methods are reported in accordance with the ARRIVE guidelines for the reporting of animal experiments. Regarding the biological plant material used in the present study, it was not collected in the wild, so it was not deposited in an herbarium or public collection, as Resveratrol was purchased in a food supplement formulation under the trade name Resveratrol (General Nutrition Centers GNC Laboratories, Pittsburgh, PA, USA; each capsule weighed 0.7 g and contained 500 mg of standardized Resveratrol extract. These laboratories authenticated the contents of the container as Resveratrol under batch number VA3020; therefore, it was not authenticated by a taxonomist, and a voucher was not needed. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- FAOSTAT. Food and Agriculture Organization of the United Nations. Cultivos y productos de ganadería. http://www.fao.org/faostat/es/#data/QCL/visualize. (Accessed on 20 September 2024).
-
- Jørgensen K, Morant AV, Morant M, et al. Biosynthesis of the cyanogenic glucosides Linamarin and Lotaustralin in Cassava: isolation, biochemical characterization, and expression pattern of CYP71E7, the oxime-metabolizing cytochrome P450 enzyme. Plant Physiol. 2011;155(1):282–92. 10.1104/pp.110.164053. - DOI - PMC - PubMed
-
- Tshala-Katumbay D, Banea-Mayambu JP, Kazadi-Kayembe T, et al. Neuroepidemiology of Konzo a spastic para-tetraparesis of acute onset in a new area of the Democratic Republic of congo. J Neurol Sci. 2001;20(1). 10.4314/ajns.v20i1.7520
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

