Epigenetic regulation of histone modifications in glioblastoma: recent advances and therapeutic insights
- PMID: 40450300
- PMCID: PMC12125905
- DOI: 10.1186/s40364-025-00788-w
Epigenetic regulation of histone modifications in glioblastoma: recent advances and therapeutic insights
Abstract
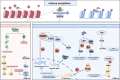
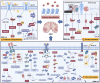
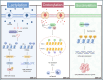
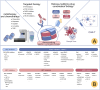
Glioblastoma (GBM) is the most common primary malignant brain tumor, characterized by its aggressive behavior, limited treatment options, and poor prognosis. Despite advances in surgery, radiotherapy, and chemotherapy, the median survival of GBM patients remains disappointingly short. Recent studies have underscored the critical role of histone modifications in GBM malignant progression and therapy resistance. Histones, protein components of chromatin, undergo various modifications, including acetylation and methylation. These modifications significantly affect gene expression, thereby promoting tumorigenesis and resistance to therapy. Targeting histone modifications has emerged as a promising therapeutic approach. Numerous pre-clinical studies have evaluated histone modification agents in GBM, including histone deacetylase inhibitors and histone methyltransferase inhibitors. These studies demonstrate that modulating histone modifications can alter gene expression patterns, inhibit tumor growth, induce apoptosis, and sensitize tumor cells to conventional treatments. Some agents have advanced to clinical trials, aiming to translate preclinical efficacy into clinical benefit. However, clinical outcomes remain suboptimal, as many agents fail to significantly improve GBM patient prognosis. These challenges are attributed to the complexity of histone modification networks and the adaptive responses of the tumor microenvironment. This review provides a comprehensive overview of epigenetic regulation mechanisms involving histone modifications in GBM, covering their roles in tumor development, tumor microenvironment remodeling, and therapeutic resistance. Additionally, the review discusses current clinical trials targeting histone modifications in GBM, highlighting successes, limitations, and future perspectives.
Keywords: Glioblastoma; Glioblastoma chemoresistance; Histone acetylation; Histone methylation; Histone modifications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: No ethics approval was required for this review that did not involve patients or patient data. Consent for publication: All authors consent to publication. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
The Impact of Epigenetic Modifications on Adaptive Resistance Evolution in Glioblastoma.Int J Mol Sci. 2021 Aug 3;22(15):8324. doi: 10.3390/ijms22158324. Int J Mol Sci. 2021. PMID: 34361090 Free PMC article. Review.
-
Targeting post-translational histone modifying enzymes in glioblastoma.Pharmacol Ther. 2021 Apr;220:107721. doi: 10.1016/j.pharmthera.2020.107721. Epub 2020 Nov 2. Pharmacol Ther. 2021. PMID: 33144118 Review.
-
Krüppel-like factor 9 and histone deacetylase inhibitors synergistically induce cell death in glioblastoma stem-like cells.BMC Cancer. 2018 Oct 22;18(1):1025. doi: 10.1186/s12885-018-4874-8. BMC Cancer. 2018. PMID: 30348136 Free PMC article.
-
The epigenetic mechanisms involved in the treatment resistance of glioblastoma.Cancer Drug Resist. 2025 Mar 13;8:12. doi: 10.20517/cdr.2024.157. eCollection 2025. Cancer Drug Resist. 2025. PMID: 40201311 Free PMC article. Review.
-
Interplay of m6 A and histone modifications contributes to temozolomide resistance in glioblastoma.Clin Transl Med. 2021 Sep;11(9):e553. doi: 10.1002/ctm2.553. Clin Transl Med. 2021. PMID: 34586728 Free PMC article.
Cited by
-
Transcriptomic HEPN1 signatures predict treatment response in low grade glioma.Discov Oncol. 2025 Aug 15;16(1):1561. doi: 10.1007/s12672-025-03395-1. Discov Oncol. 2025. PMID: 40815398 Free PMC article.
References
-
- Alexander BM, Cloughesy TF. Adult Glioblastoma. J Clin Oncol : Off J Am Soc Clin Oncol. 2017;35(21):2402–9. - PubMed
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

