Neurodegeneration models in Parkinson's disease: cellular and molecular paths to neuron death
- PMID: 40450319
- PMCID: PMC12125839
- DOI: 10.1186/s12993-025-00279-w
Neurodegeneration models in Parkinson's disease: cellular and molecular paths to neuron death
Abstract
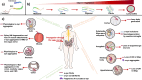
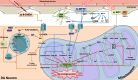
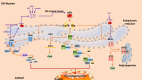
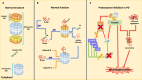
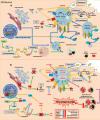
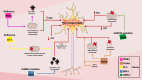
Parkinson's disease (PD) is a progressive neurodegenerative disorder that affects dopaminergic neurons in the substantia nigra pars compacta. It is a complex disease that is strongly influenced by environmental and genetic factors. While the exact causes of PD are not well understood, research on the effects of toxic substances that induce neuronal death has shed some light on the etiology of the disease. In addition, studies have implicated protein aggregation and impaired mitochondrial, endoplasmic reticulum (ER), proteasome, and/or lysosomal function in the pathogenesis of PD. This review focuses on the alterations in intraneuronal organelles and the role of toxic agents that lead to organelle damage and neurodegeneration that characterize PD. We describe in vivo and in vitro models that have been used to elucidate the factors that lead to the death of dopaminergic neurons and summarize the molecular mechanisms that may underlie the changes that promote neurodegeneration. A deeper understanding of the mechanisms of neuronal death may help us to develop new therapies and interventions to delay or prevent the progression of PD.
Keywords: Dopaminergic neuron; Molecular mechanism; Neurodegeneration; Neuronal death; Parkinson disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Reduced expression of Pss gene in Drosophila cortex glia causes dopaminergic cell death.J Parkinsons Dis. 2025 Aug;15(5):957-969. doi: 10.1177/1877718X251349407. Epub 2025 Jun 16. J Parkinsons Dis. 2025. PMID: 40518954
-
Parkinson-like wild-type superoxide dismutase 1 pathology induces nigral dopamine neuron degeneration in a novel murine model.Acta Neuropathol. 2025 Mar 5;149(1):22. doi: 10.1007/s00401-025-02859-6. Acta Neuropathol. 2025. PMID: 40042537 Free PMC article.
-
Neuromelanin: Its role in the pathogenesis of idiopathic Parkinson's disease and potential as a therapeutic target.Parkinsonism Relat Disord. 2023 Jul;112:105448. doi: 10.1016/j.parkreldis.2023.105448. Epub 2023 May 19. Parkinsonism Relat Disord. 2023. PMID: 37236833 Review.
-
DDAH-1 maintains endoplasmic reticulum-mitochondria contacts and protects dopaminergic neurons in Parkinson's disease.Cell Death Dis. 2024 Jun 7;15(6):399. doi: 10.1038/s41419-024-06772-w. Cell Death Dis. 2024. PMID: 38849335 Free PMC article.
-
Berberine's paradox in Neurodegeneration: Therapeutic promise and safety challenges in Parkinson's disease.Neuropharmacology. 2025 Nov 1;278:110555. doi: 10.1016/j.neuropharm.2025.110555. Epub 2025 Jun 6. Neuropharmacology. 2025. PMID: 40482960 Review.
References
-
- Shibasaki Y, Baillie DAM, Clair D, Brookes AJ. High-resolution mapping of SNCA encoding α-synuclein, the non-Aβ component of Alzheimer’s disease amyloid precursor, to human chromosome 4q21.3→q22 by fluorescence in situ hybridization. Cytogenet Genome Res. 1995;71:54–5. 10.1159/000134061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

