Pasritamig, a First-in-Class, Bispecific T-Cell Engager Targeting Human Kallikrein 2, in Metastatic Castration-Resistant Prostate Cancer: A Phase I Study
- PMID: 40450573
- PMCID: PMC12288886
- DOI: 10.1200/JCO-25-00678
Pasritamig, a First-in-Class, Bispecific T-Cell Engager Targeting Human Kallikrein 2, in Metastatic Castration-Resistant Prostate Cancer: A Phase I Study
Abstract
Purpose: We report phase I trial results for pasritamig, a first-in-class, T-cell-engaging bispecific antibody targeting human kallikrein 2 (KLK2) expressed on the surface of prostate cancer (PC) cells.
Methods: Participants had metastatic castration-resistant PC and ≥1 prior therapy. Pasritamig was escalated from 0.5 mg to 2,000 mg for subcutaneous administration and from 150 mg to 900 mg for intravenous (IV) administration at dosing frequencies ranging from once every week to once every 6 weeks with different step-up dosing schedules. The primary objectives were to determine safety and the recommended phase II dose (RP2D) of pasritamig. Secondary objectives included preliminary assessment of antitumor activity.
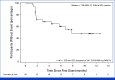
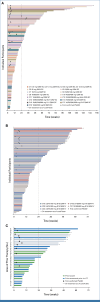
Results: One hundred seventy-four participants received pasritamig, with a median of 4 prior lines of systemic therapy. Treatment-related adverse events (TRAEs) occurred in 144 of 174 (82.8%) participants, with 17 of 174 (9.8%) experiencing grade ≥3 TRAEs. The RP2D was determined to be 3.5 mg (day 1), 18 mg (day 8), 300 mg (day 15), and then 300 mg IV once every 6 weeks. In the RP2D safety population (n = 45), infusion-related reactions (11/45, 24.4%), fatigue (7/45, 15.6%), cytokine release syndrome (CRS; 4/45, 8.9%, all grade 1), and lipase increase (4/45, 8.9%) were the most frequent TRAEs; all were grade 1 or 2. In the RP2D efficacy population (n = 33), median radiographic progression-free survival was 7.85 (95% CI, 2.89 to not estimable) months, and 14 of 33 (42.4%) participants achieved a ≥50% decrease from baseline in prostate-specific antigen.
Conclusion: Pasritamig demonstrated a favorable safety profile with very low rates of CRS and could be safely administered in an outpatient setting. Preliminary antitumor activity demonstrated proof of concept for KLK2 as a target in PC, warranting further development of pasritamig.
Trial registration: ClinicalTrials.gov NCT04898634.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

