A Matching-Adjusted Indirect Comparison of Guselkumab and Secukinumab in Patients with Psoriatic Arthritis Over 52 Weeks
- PMID: 40450642
- PMCID: PMC12246347
- DOI: 10.1007/s40744-025-00771-9
A Matching-Adjusted Indirect Comparison of Guselkumab and Secukinumab in Patients with Psoriatic Arthritis Over 52 Weeks
Abstract
Introduction: Studies evaluating the long-term comparative efficacy between biologic therapies for psoriatic arthritis (PsA) are scarce. Two biologic therapies, guselkumab and secukinumab, were evaluated up to 52 weeks in a mixed patient population (biologic-naïve and biologic-experienced patients).
Methods: An unanchored matching-adjusted indirect comparison (MAIC) was conducted to compare guselkumab 100 mg every 8 weeks (Q8W) and every 4 weeks (Q4W) versus secukinumab 150 mg Q4W and 300 mg Q4W on American College of Rheumatology (ACR) and Psoriasis Area and Severity Index (PASI) responses from weeks 4 through 52 using pooled individual patient-level data from guselkumab trials (COSMOS, DISCOVER-1 and -2) and pooled summary-level data from secukinumab trials (FUTURE 2, 3, 4, and 5). For the primary analysis, patients from the guselkumab trials were re-weighted on six clinically relevant baseline characteristics to match those in the secukinumab trials. Additional characteristics were included as a sensitivity analysis. A scenario analysis was conducted in a biologic-naïve patient population only.
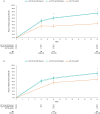
Results: For the mixed population, both guselkumab doses initially had numerically or significantly lower ACR 20 responses than both secukinumab doses prior to weeks 12-20; however, from weeks 12-24 onward, ACR 20 responses became numerically or significantly higher for guselkumab. For PASI 90 responses, both guselkumab doses showed significantly higher responses than both secukinumab doses at weeks 24 and 52. Notably, at 52 weeks, ACR 20 and PASI 90 responses for both doses of guselkumab were numerically or significantly higher than both doses of secukinumab. Results from the sensitivity and scenario analyses were similar to the primary analysis.
Conclusions: While the IL-17A inhibitor secukinumab may demonstrate more rapid and greater efficacy before weeks 12-20, both doses of guselkumab provide similar or greater efficacy on joint and skin outcomes compared to both doses of secukinumab from week 24 onward. This study provides valuable insights for treatment decisions when considering the chronic nature of PsA.
Keywords: Guselkumab; Matching-adjusted indirect comparison; Psoriatic arthritis; Secukinumab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Suzy van Sanden, Agata Schubert, Miriam Zimmermann, and Fareen Hassan are employees of Johnson & Johnson Innovative Medicine. Barkha P. Patel is an employee of EVERSANA, which received funding from Johnson & Johnson Innovative Medicine for this study. Ethical Approval: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Figures



References
-
- Monteleone G, Moscardelli A, Colella A, Marafini I, Salvatori S. Immune-mediated inflammatory diseases: common and different pathogenic and clinical features. Autoimmun Rev. 2023;22(10): 103410. - PubMed
-
- Marzo-Ortega H, Packham J, Pujades-Rodriguez M. “Too much of a good thing”: can network meta-analysis guide treatment decision-making in psoriatic arthritis? Rheumatology (Oxford). 2021;60(7):3042–4. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

