Myristoylation of TMEM106B by NMT1/2 regulates TMEM106B trafficking and turnover
- PMID: 40451428
- PMCID: PMC12268641
- DOI: 10.1016/j.jbc.2025.110322
Myristoylation of TMEM106B by NMT1/2 regulates TMEM106B trafficking and turnover
Abstract
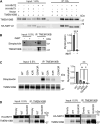
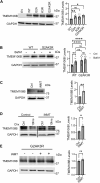
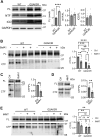
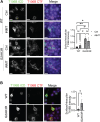
TMEM106B, a type II transmembrane protein localized on the lysosomal membrane, has been identified as a central player in neurodegeneration and brain aging during the past decade. TMEM106B variants that increase TMEM106B expression levels are linked to several neurodegenerative diseases, including frontotemporal lobar degeneration (FTLD). Additionally, the C-terminal lumenal fragment of TMEM106B was recently shown to form amyloid fibrils during aging and neurodegeneration. However, the mechanisms regulating TMEM106B levels are not well understood. Here we show that TMEM106B is myristoylated by NMT1/2 enzymes at its glycine 2 α-amino group and its lysine 3 ε-amino group. Myristoylation decreases TMEM106B levels by promoting its lysosomal degradation. Furthermore, we demonstrate that TMEM106B C-terminal fragments (CTFs) can be detected under physiological conditions, and the levels of CTFs are regulated by myristoylation and lysosomal activities. In addition, we show that non-myristoylated TMEM106B accumulates on the cell surface, indicating that myristoylation affects TMEM106B trafficking within the cell. Taken together, these findings suggest that TMEM106B myristoylation is an important mechanism regulating its function, trafficking, and turnover.
Keywords: NMT1/2; TMEM106B; lysosome; myristoylation; neurodegeneration; protein processing; protein trafficking.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: H. L. is a founder and consultant for Sedec Therapeutics. AY, BG, IP, MAYC, and NFH are current or former employees of Alector, LLC and may have an equity interest in Alector, Inc. Several authors have patents related to TMEM106B-specific antibodies.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

