Performance of malaria rapid diagnostic test, microscopy, polymerase chain reaction, and histopathology to diagnose malaria among pregnant and parturient women using peripheral, placental, and cord blood, and placental biopsy in Majang Zone of Gambella Region, Southwest Ethiopia
- PMID: 40452013
- PMCID: PMC12128499
- DOI: 10.1186/s12936-025-05426-2
Performance of malaria rapid diagnostic test, microscopy, polymerase chain reaction, and histopathology to diagnose malaria among pregnant and parturient women using peripheral, placental, and cord blood, and placental biopsy in Majang Zone of Gambella Region, Southwest Ethiopia
Abstract
Background: Accurate, reliable, and timely diagnosis is essential for mitigating malaria in pregnancy (MiP) and its adverse outcomes. This study aimed to evaluate the accuracy of malaria diagnostic tests for detecting Plasmodium infection in peripheral, placental, and cord blood and placental biopsy in the Majang Zone of Gambella Region, Southwest Ethiopia.
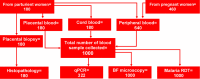
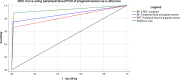
Methods: A cross-sectional study involving 640 (460 pregnant and 180 parturient) women visiting five public health facilities for antenatal care and delivery services in Majang Zone was conducted from November 2022 to March 2023. Peripheral, placental, and cord blood were collected to detect Plasmodium infection by rapid diagnostic test (RDT), microscopy, and quantitative Polymerase Chain Reaction (qPCR). Placental biopsy was collected for placental malaria (PM) diagnosis by histopathology. Performance indices, Kappa Coefficient, and Receiver Operating Characteristic were determined using Statistical Package for Social Science Version 26.0, Microsoft Excel Version 19.0, and Stata Version 17.0.
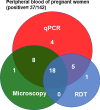
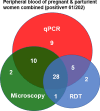
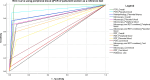
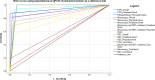
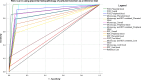
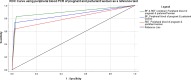
Results: One thousand blood (640 peripheral, 180 placental, and 180 cord) and 180 placental biopsy specimens collected from pregnant and parturient women were analysed in this study. Malaria positivity rate among pregnant and parturient women was 21.1% and 28.9%, respectively. Considering peripheral blood qPCR as a reference, the sensitivity, specificity, accuracy, and agreement of RDT were (63.5%, 93.0%, 0.807, and 0.683), and microscopy were (73.1%, 98.0%, 0.855, and 0.764) to detect Plasmodium infection in combined peripheral blood of pregnant and parturient women, respectively. Considering placental blood qPCR as a reference, the sensitivity, specificity, accuracy, and agreement of RDT were (56.3%, 95.5%, 0.759, and 0.574), microscopy were (81.3%, 97.7%, 0.895, and 0.822), and histopathology (87.5%, 100.0%, 0.892, and 0.911) to detect Plasmodium infection in placental blood of parturient women, respectively. Considering placental histopathology a as reference, the sensitivity, specificity, accuracy, and agreement of RDT were (56.8%, 97.1%, 0.753, and 0.609), microscopy were (68.2%, 98.5%, 0.918, and 0.735), and qPCR (100.0%, 95.7%, 0.978, and 0.911) to detect Plasmodium infection in placental blood of parturient women, respectively.
Conclusion: Diagnostic performance of RDT and microscopy was sub-optimal to detect Plasmodium infection among pregnant and parturient women. More sensitive diagnostic tests are needed to mitigate MiP.
Keywords: Diagnostic test accuracy; Ethiopia; Gambella; Histopathology; Kappa; Majang; Malaria in pregnancy; Microscopy; Placental malaria; QPCR; RDT; Receiver operating characteristic; Sensitivity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted with the approval of the relevant Ethical Committee Review as well as prior written informed consent for each participant. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- WHO. A framework for malaria elimination. Geneva: World Health Organization; 2017.
-
- Desai M, Hill J, Fernandes S, Walker P, Pell C, Gutman J, et al. Prevention of malaria in pregnancy. Lancet Infect Dis. 2018;18:e119–32. - PubMed
-
- WHO. Guidelines for malaria. Geneva: World Health Organization; 2023.
-
- Rogerson SJ, Desai M, Mayor A, Sicuri E, Taylor SM, van Eijk AM. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis. 2018;18:e107–18. - PubMed
-
- WHO. Malaria microscopy quality assurance manual- Version 2. Geneva: World Health Organization; 2016.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

