British Society of Echocardiography guideline for the transthoracic echocardiographic assessment of adult patients with obstructive hypertrophic cardiomyopathy receiving myosin-inhibitor therapy
- PMID: 40452068
- PMCID: PMC12128337
- DOI: 10.1186/s44156-025-00078-z
British Society of Echocardiography guideline for the transthoracic echocardiographic assessment of adult patients with obstructive hypertrophic cardiomyopathy receiving myosin-inhibitor therapy
Keywords: Mavacamten; Myosin-inhibitor; Obstructive HCM; Surveillance Echocardiogram.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

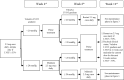

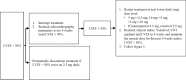
References
-
- NICE. Mavacamten for treating symptomatic obstructive hypertrophic cardiomyopathy. 2023. https://www.nice.org.uk/guidance/ta913 - PubMed
-
- Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, De Winter T, Elliott PM, Flather M, Garcia-Pavia P, Haugaa KH, Ingles J, Jurcut RO, Klaassen S, Limongelli G, Loeys B, Mogensen J, Olivotto I, Pantazis A, Sharma S, Peter Van Tintelen J, Ware JS, Kaski JP, ESC Scientific Document Group. 2023 ESC guidelines for the management of cardiomyopathies: developed by the task force on the management of cardiomyopathies of the European society of cardiology (ESC). Eur Heart J. 2023;44(37):3503–626. 10.1093/eurheartj/ehad194. - DOI - PubMed
-
- Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2020;142(25):e558–631. 10.1161/CIR.0000000000000937. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

