Dupilumab Treatment Is Associated With Clinical Improvement and a Shift Toward a Health-Associated Nasal Passage Microbiota in Diffuse Type 2 Chronic Rhinosinusitis
- PMID: 40452334
- PMCID: PMC12186592
- DOI: 10.1111/all.16600
Dupilumab Treatment Is Associated With Clinical Improvement and a Shift Toward a Health-Associated Nasal Passage Microbiota in Diffuse Type 2 Chronic Rhinosinusitis
Abstract
Background: Nasal microbiota composition of patients with diffuse type 2 chronic rhinosinusitis with nasal polyps (CRSwNP) is altered compared to healthy individuals. Dupilumab, an anti-IL-4Rα-mAb, modulates type 2 inflammation, but the effect on microbiota composition in CRSwNP is unknown. The aim of this study was to investigate longitudinal effects of dupilumab on the nasal passage and gastrointestinal microbiota in patients with diffuse type 2 CRSwNP.
Methods: Twenty-seven patients with diffuse type 2 CRSwNP treated with dupilumab 300 mg subcutaneously every 2 weeks, 10 untreated patients with CRSwNP, and 11 healthy controls were included. Nasal and stool samples were collected at Days 0, 28, 90, and 180 posttreatment of the treated CRSwNP group and at Days 0 and 28 of untreated CRSwNP and healthy controls. The samples were analyzed using 16S rRNA gene amplicon sequencing (V3/V4).
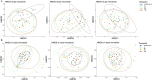
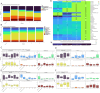
Results: In CRSwNP patients, the most abundant genera in nasal passage microbiota were Corynebacterium and Staphylococcus. Cutibacterium and Lawsonella were less abundant in CRSwNP at baseline compared to healthy controls. Dupilumab treatment was associated with increased relative abundances in the nasal passage of genera such as Lawsonella, Corynebacterium, and Dolosigranulum. Microbial diversity of the gastrointestinal microbiota in CRSwNP at baseline was significantly higher than in healthy controls. There were no changes in gastrointestinal microbiota during dupilumab treatment.
Conclusion: Dupilumab treatment was associated with a shift in the nasal passage bacterial microbiota toward that of healthy controls, whereas the composition of gastrointestinal microbiota did not change. These findings suggest that nasal passage microbiota composition is influenced by the underlying inflammatory endotype.
Keywords: CRSwNP; biologicals; dupilumab; microbiome; microbiota; type 2 inflammation.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
M.B.S. is a consultant for Sanofi, GSK, Novartis, Astra Zeneca, and MSD unrelated to this study. U.C.S. is a consultant for Sanofi, GSK, and Astra Zeneca unrelated to this study. The other authors reported no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

