Evaluating the feasibility of gene replacement strategies to treat MTRFR deficiency
- PMID: 40452409
- PMCID: PMC12171093
- DOI: 10.1242/dmm.052120
Evaluating the feasibility of gene replacement strategies to treat MTRFR deficiency
Abstract
Mitochondrial translation release factor in rescue (MTRFR) catalyzes a termination step in protein synthesis, facilitating release of the nascent chain from mitoribosomes. Pathogenic variants in MTRFR cause MTRFR deficiency and are loss-of-function variants. Here, we tested gene replacement as a possible therapeutic strategy. A truncating mutation (K155*) was generated in mice; however, homozygotes die embryonically whereas mice heterozygous for this K155* allele are normal. We also generated transgenic strains expressing either wild-type human MTRFR or a partially functional MTRFR. Despite dose-dependent phenotypes from overexpression in vitro, neither transgene caused adverse effects in vivo. In K155* homozygous mice, the wild-type MTRFR transgene completely rescued the phenotype with only one copy present, whereas the mutant transgene rescued less efficiently. Detailed evaluation of mice rescued with the wild-type MTRFR transgene revealed no abnormalities. In human induced pluripotent stem cell (hiPSC)-derived knockdown neurons, mitochondrial phenotypes were corrected by AAV9-mediated delivery of MTRFR. Thus, we find no toxicity from truncated gene products or overexpression of MTRFR in vivo, and expression of MTRFR corrects phenotypes in both mouse and hiPSC models.
Keywords: C12ORF65; Behr's syndrome; CMT6; Leigh syndrome; MRPL58; Mitochondrial translation.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
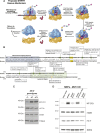
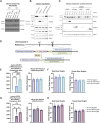

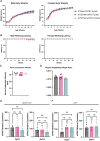
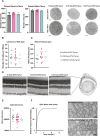

References
-
- Antonicka, H., Ostergaard, E., Sasarman, F., Weraarpachai, W., Wibrand, F., Pedersen, A. M., Rodenburg, R. J., Van Der Knaap, M. S., Smeitink, J. A., Chrzanowska-Lightowlers, Z. M.et al. (2010). Mutations in C12orf65 in patients with encephalomyopathy and a mitochondrial translation defect. Am. J. Hum. Genet. 87, 115-122. 10.1016/j.ajhg.2010.06.004 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- Muscular Dystrophy UK
- LifeArc Centre to Treat Mitochondrial Diseases
- National Ataxia Foundation
- Action for A-T
- Helsingin Yliopisto
- R37 NS054154/NS/NINDS NIH HHS/United States
- Research Council of Finland
- Hereditary Disease Foundation
- BRC-1215-20014/NIHR Cambridge Biomedical Research Centre
- Sigrid Juséliuksen Säätiö
- UK Research and Innovation
- CA34196/CA/NCI NIH HHS/United States
- MR/S005021/1/MRC_/Medical Research Council/United Kingdom
- MR/V009346/1/MRC_/Medical Research Council/United Kingdom
- Magnus Ehrnrooth Foundation
- R37NS054154/NS/NINDS NIH HHS/United States
- Lindsey Flynt
- 226653/Z/22/Z/WT_/Wellcome Trust/United Kingdom
- Jackson Laboratory
- 101120256: MMM/HORIZON EUROPE Marie Sklodowska-Curie Actions
- P30 CA034196/CA/NCI NIH HHS/United States
- Ataxia UK
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

