Functional multiomics reveals genetic and pharmacologic regulation of surface CD38 in multiple myeloma
- PMID: 40453054
- PMCID: PMC12093323
- DOI: 10.1016/j.bneo.2024.100025
Functional multiomics reveals genetic and pharmacologic regulation of surface CD38 in multiple myeloma
Abstract
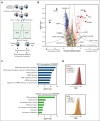
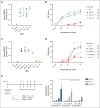
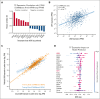
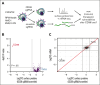
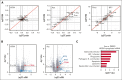
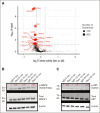
CD38 is a surface ectoenzyme expressed at high levels on myeloma plasma cells and is the target for the monoclonal antibodies (mAbs) daratumumab and isatuximab. Pretreatment CD38 density on tumor cells is an important determinant of mAb efficacy. Several small molecules have been found to increase tumor surface CD38, with the goal of boosting mAb efficacy in a cotreatment strategy. Numerous other CD38-targeting therapeutics are currently in preclinical or clinical development. Here, we sought to extend our currently limited insight into CD38 surface expression by using a multiomics approach. Genome-wide CRISPR interference screens integrated with patient-centered epigenetic analysis confirmed known regulators of CD38, such as RARA, while revealing XBP1 and SPI1 as other key transcription factors governing surface CD38 levels. CD38 knockdown followed by cell surface proteomics demonstrated no significant remodeling of the myeloma "surfaceome" after genetically induced loss of this antigen. Integrated transcriptome and surface proteome data confirmed high specificity of all-trans retinoic acid in upregulating CD38, in contrast to the broader effects of azacytidine and panobinostat. Finally, unbiased phosphoproteomics identified inhibition of MAP kinase pathway signaling in tumor cells after daratumumab treatment. Our work provides a resource to design strategies to enhance efficacy of CD38-targeting immunotherapies in myeloma.
© 2024 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.C. is a shareholder of Genentech/Roche. P.R. is a shareholder of Senti Biosciences. A.P.W. is an equity holder and scientific advisory board member of Indapta Therapeutics, LLC and Protocol Intelligence, LLC. M.K. has filed a patent application related to CRISPRi screening (US patent number PCT/US15/40449); and serves on the scientific advisory boards of Engine Biosciences, Cajal Neuroscience, and Casma Therapeutics. The remaining authors declare no competing financial interests. The current affiliation for P.C. is Genentech/Roche, South San Francisco, CA. The current affiliation for P.R. is Senti Biosciences, South San Francisco, CA.
Figures

References
-
- Syed YY. Daratumumab: a review in combination therapy for transplant-ineligible newly diagnosed multiple myeloma. Drugs. 2019;79(4):447–454. - PubMed
-
- Bonello F, D'Agostino M, Moscvin M, Cerrato C, Boccadoro M, Gay F. CD38 as an immunotherapeutic target in multiple myeloma. Expert opinion on biological therapy. 2018;18(12):1209–1221. - PubMed
-
- Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–970. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

