Real-world experience with targeted therapy in patients with histiocytic neoplasms in the Netherlands and in Belgium
- PMID: 40453055
- PMCID: PMC12096411
- DOI: 10.1016/j.bneo.2024.100023
Real-world experience with targeted therapy in patients with histiocytic neoplasms in the Netherlands and in Belgium
Abstract
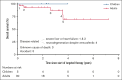
Histiocytic disorders are rare hematologic neoplasms characterized by a notable dependence on mitogen-activated protein kinase signaling. Targeted therapy is an emerging treatment option, yet the number of reported patients remains limited. Here, we describe 40 patients with histiocytic neoplasms who were treated with targeted therapy in 7 tertiary referral hospitals from the Netherlands and Belgium. The cohort comprised of 6 (15%) children and 34 (85%) adults with diverse histiocytoses, including Langerhans cell histiocytosis (LCH; n = 12), Erdheim-Chester disease (n = 14), central nervous system xanthogranuloma (n = 2), Rosai-Dorfman disease (n = 3), histiocytic sarcoma (n = 2), ALK-positive histiocytosis (n = 1), and mixed/unclassifiable histiocytosis (n = 6). Five patients were included in a clinical trial; 35 (88%) received BRAF/MEK inhibitors outside of trials. Among these 35 patients with available follow-up data, median time on targeted treatment was 1.9 years (range, 0.04-5.8 years). Complete or partial responses were observed in 25 of 27 (93%) patients treated for multisystemic and/or solid lesions and 2 of 8 (25%) patients treated for neurodegenerative LCH. Responses were generally durable, although 10 patients lost response after dose reduction or therapy interruption. Responses were recaptured in 9 of 10 cases. Two patients developed new or progressive neurodegenerative lesions: 1 during and 1 after vemurafenib therapy. At last follow-up, 8 adults had stopped targeted therapy because of toxicity. This study corroborates the favorable outcomes of BRAF/MEK inhibition in patients with histiocytosis described previously. However, it also highlights limitations and calls for prospective studies.
© 2024 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: S.H.T. received financial compensation from Roche for a review of the American Society of Hematology Annual Meeting and Exposition. P.S. reports consulting/advisory roles for Deciphera, Ellipses Pharma, Transgene, Exelixis, Boehringer Ingelheim, Studiecentrum voor Kernenergie, Adcendo, PharmaMar, Merck Healthcare KGaA, Medpace, Cogent Biosciences, Eisai, Curio Science, LLX Solutions, Servier, Genmab, Sanofi, Regeneron, Moleculin Biotech, Avacta Life Sciences, Amryt Pharma, Union Chimique Belge, Boxer Capital, NEC OncoImmunity AS, Sonata Therapeutics, IDRx, and Telix Pharmaceuticals; and institutional research funding from CoBioRes NV, Eisai, G1 Therapeutics, PharmaMar, Genmab, Merck, Sartar Therapeutics, ONA Therapeutics, and Adcendo. The remaining authors declare no competing financial interests. A complete list of the members of the Dutch-Belgian Cooperative Trial Group for Hematology Oncology Histiocytic and Lymphocytic Diseases Working Group appears in “Appendix.”
Figures






References
-
- Kemps PG, Kester L, Scheijde-Vermeulen MA, et al. Demographics and additional haematologic cancers of patients with histiocytic/dendritic cell neoplasms. Histopathology. 2024;84(5):837–846. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

