Response-guided first-line therapy and treatment of relapse in aggressive lymphoma: 10-year follow-up of the PETAL trial
- PMID: 40453056
- PMCID: PMC12082160
- DOI: 10.1016/j.bneo.2024.100018
Response-guided first-line therapy and treatment of relapse in aggressive lymphoma: 10-year follow-up of the PETAL trial
Abstract
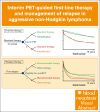
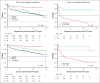
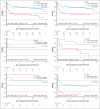
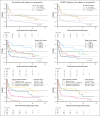
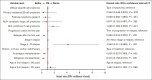
The PETAL (Positron Emission Tomography-Guided Therapy of Aggressive Non-Hodgkin Lymphomas) trial investigated whether treatment intensification after 2 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP; plus rituximab [R] for CD20+ lymphomas) improved survival in aggressive non-Hodgkin lymphoma. A total of 754 patients (87.5%) had a negative and 108 (12.5%) had a positive interim positron emission tomography (iPET) scan. iPET-positive patients were randomly assigned to receive another 6 (R-)CHOP cycles or 6 blocks of a more intensive protocol. Interim PET-negative patients received another 4 cycles of R-CHOP with or without 2 additional doses of R. After a median follow-up of 4 years, treatment intensification had not improved outcome. These results were confirmed after 10.3 years of follow-up. The present analysis also describes the management of relapse that was part of the study. Among 240 relapsing patients, 94 of 133 autologous transplantation-eligible patients (70.7%) and 16 of 107 ineligible patients (15.0%) received the salvage treatments recommended in the study protocol. Adherence to recommendations had no impact on outcome. Best results were seen after allogeneic transplantation, followed by autologous transplantation and treatment without transplantation (5-year overall survival rate, 64.3% vs 45.5% vs 22.6%), but patients undergoing allogeneic transplantation were significantly younger and their disease was well controlled at the time of transplantation. Early outcome prediction by iPET, alone or in combination with other methods, is a powerful tool to investigate the value of immunological treatment options for patients with a poor response to chemotherapy. This trial was registered at ClinicalTrials.gov as #NCT00554164.
© 2024 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests. A complete list of the members of the PETAL (Positron Emission Tomography–Guided Therapy of Aggressive Non-Hodgkin Lymphomas) trial investigators appears in the supplemental Appendix.
Figures

References
-
- Cottereau A-S, El-Galaly TC, Becker S, et al. Predictive value of PET response combined with baseline metabolic tumor volume in peripheral T-cell lymphoma patients. J Nucl Med. 2018;59(4):589–595. - PubMed
-
- Dührsen U, Müller S, Hertenstein B, et al. Positron Emission Tomography-Guided Therapy of Aggressive Non-Hodgkin Lymphomas (PETAL): a multicenter, randomized phase III trial. J Clin Oncol. 2018;36(20):2024–2034. - PubMed
-
- Rekowski J, Hüttmann A, Schmitz C, et al. Interim PET evaluation in diffuse large B-cell lymphoma employing published recommendations: comparison of the Deauville 5-point scale and the ΔSUVmax method. J Nucl Med. 2021;62(1):37–42. - PubMed
-
- Lin C, Itti E, Haioun C, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48(10):1626–1632. - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

