Real-world outcomes of newly diagnosed AML treated with venetoclax and azacitidine or low-dose cytarabine in the UK NHS
- PMID: 40453057
- PMCID: PMC12082122
- DOI: 10.1016/j.bneo.2024.100017
Real-world outcomes of newly diagnosed AML treated with venetoclax and azacitidine or low-dose cytarabine in the UK NHS
Abstract
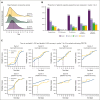
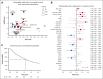
Venetoclax with azacitidine is the standard of care for patients with acute myeloid leukemia (AML) who are unfit for intensive chemotherapy; however, uncertainties remain regarding the treatment schedule, accurate prognostication, and outcomes for patients treated outside clinical trials. The option of venetoclax with low-dose cytarabine (LDAC) is also available; however, it is not clear for which patients it may be a useful alternative. Here, we report a large real-world cohort of 654 patients treated in 53 UK hospitals with either venetoclax and azacitidine (n = 587) or LDAC (n = 67). The median age was 73 years, and 59% had de novo AML. Most patients received 100 mg of venetoclax with an azole antifungal. In cycle 1, patients spent a median of 14 days in the hospital, and 85% required red cell transfusion, 59% platelet transfusion, and 63% required IV antibiotics. Supportive care requirements significantly reduced after the first cycle. Patients receiving venetoclax-azacitidine had a complete remission (CR)/CR with incomplete hematological recovery rate of 67%, day 30 and day 60 mortality of 5% and 8%, respectively, and median overall survival of 13.6 months. Mutations in NPM1, RUNX1, STAG2, and IDH2 were associated with improved survival, whereas age, secondary and therapy-related AML, +8, MECOM rearrangements, complex karyotype, ASXL1, and KIT mutations were associated with poorer survival. Prognostic systems derived specifically for patients treated with venetoclax-azacitidine performed better than the European LeukemiaNet and Medical Research Council classifications; however, improved risk classifications are still required. In the 149 patients with NPM1 mutated AML, outcomes were similar for those treated with venetoclax-azacitidine and venetoclax-LDAC.
© 2024 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J. Othman declares honoraria from Astellas and Jazz Pharmaceuticals; and speaker’s fees and advisory board fees from Pfizer, Jazz Pharmaceuticals, Astellas, and AbbVie. P.G. declares honoraria from Astellas. F.H. declares meeting sponsorship and honoraria from AbbVie. R. Dang declares meeting sponsorship from Jazz; and honoraria from AbbVie. J.V. declares meeting support from BeiGene, Janssen, and Jazz; and honoraria from AbbVie and AstraZeneca. P. Krishnamurthy declares honoraria from Jazz, Astellas, and Gilead; speaker’s bureau with Astellas; and consultancy for Jazz and Gilead. A.-L.L. declares honoraria from Astella, AbbVie, Amgen, Kite, Novartis, Jazz, and Daiichi Sankyo; and speaker’s bureau with Kite, Takeda, and Astellas. V.M. declares consultancy and honoraria from AbbVie. N.F. declares investigator meetings with Novartis and MEI Pharma. A.K. declares meeting sponsorship from Jazz, Medac, and Servier; speaker’s bureau with AbbVie, Astellas, Jazz and Servier; and consultancy/advisory board for TC BioPharm, Novartis, Synairgen, and Takeda. J. O'Nions declares honoraria from AbbVie, Astellas, Janssen, Jazz and Servier. R. Dillon declares research funding from AbbVie and Amgen; and consultancy with Astellas, Pfizer, Novartis, Jazz, BeiGene, Shattuck, and AvenCell. The remaining authors declare no competing financial interests.
Figures



References
-
- SEER Database . National Cancer Institute, Surveillance, Epidemiology, and End Results Program; 2024. Cancer Stat Facts: Leukemia – Acute Myeloid Leukemia (AML)
-
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. - PubMed
-
- Karrar O, Abdelmagid M, Rana M, et al. Venetoclax duration (14 vs. 21 vs. 28 days) in combination with hypomethylating agent in newly diagnosed acute myeloid leukemia: comparative analysis of response, toxicity, and survival. Am J Hematol. 2023;99(2):E63–E66. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

