Extracorporeal photopheresis as induction therapy in lung transplantation for cystic fibrosis: a pilot randomized trial
- PMID: 40453071
- PMCID: PMC12122324
- DOI: 10.3389/fimmu.2025.1583460
Extracorporeal photopheresis as induction therapy in lung transplantation for cystic fibrosis: a pilot randomized trial
Abstract
Introduction: Extracorporeal photopheresis (ECP) is a viable treatment that slows the progression of chronic lung allograft dysfunction. Despite its immunoregulatory potential, data on extracorporeal photopheresis as an induction therapy remain rather limited.
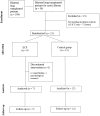
Methods: We conducted a pilot randomized controlled study on ECP as induction therapy in cystic fibrosis patients undergoing primary lung transplantation. Primary endpoints included safety, assessed based on the incidence of adverse events, treatment-related toxicity, and procedure-related complication rates; and feasibility, evaluated through the completion rate of scheduled ECP sessions, patient tolerability, and treatment discontinuation rates. Secondary endpoint consisted of an exploratory assessment of efficacy, using a composite measure that included three key components: freedom from biopsy-proven acute rejection within the first 12 months, absence of chronic lung allograft dysfunction at 36 months, and optimal graft function, defined as a predicted forced expiratory volume in the first second ≥ 90% at 36 months. Finally, exploratory endpoints included cell phenotypic and functional analyses, secreted immune protein profiling, and gene expression analysis for mechanistic insights. Patients were randomly assigned to receive either standard immunosuppressive therapy alone or standard therapy plus six sessions of extracorporeal photopheresis, with a follow-up period of 36 months.
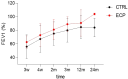
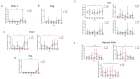
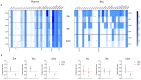
Results: Among 36 cystic fibrosis patients who underwent lung transplantation between 2018 and 2021 and met the eligibility criteria, 21 were randomized (9 to the study group and 12 to the control group). No patients in the treatment group experienced adverse events. The enrollment rate was 61%, and the treatment discontinuation rate was 22%. The clinical composite endpoint was achieved by 28.6% of patients in the treatment group and 16.7% in the control group. Exploratory endpoint analyses revealed significant decreases in pro-inflammatory cytokines, degranulating CD8+ T lymphocytes, and NK cells in the treatment group. Moreover, significant increases in Treg lymphocytes, IL-10-producing NK cells, and anti-inflammatory cytokines appeared to be associated with improved pulmonary function in the treatment group.
Conclusions: Induction therapy with extracorporeal photopheresis is safe and feasible in lung transplantation for cystic fibrosis. Some clinical benefits appear to persist for the first 36 months of follow-up. Interestingly, a correlation between immunological modulation induced by extracorporeal photopheresis and pulmonary function was observed.
Clinical trial registration: https://clinicaltrials.gov/study/NCT03500575?cond=NCT03500575&rank=1, identifier NCT03500575.
Keywords: cystic fibrosis; immunomodulation; induction therapy; lung transplanation; photopheresis.
Copyright © 2025 Righi, Fenizia, Trabattoni, Nosotti, Grisorio, Vanetti, di Tella, Mocellin, Fantini, Prati, Morlacchi, Rossetti, Blasi, Clerici and Rosso.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. . The registry of the international society for heart and lung transplantation: 29th adult lung and heart-lung transplant report—2012. J Heart Lung Transplantation. (2012) 31:1073–86. doi: 10.1016/j.healun.2012.08.004 - DOI - PubMed
-
- Treede H, Glanville AR, Klepetko W, Aboyoun C, Vettorazzi E, Lama R, et al. . Tacrolimus and cyclosporine have differential effects on the risk of development of bronchiolitis obliterans syndrome: Results of a prospective, randomized international trial in lung transplantation. J Heart Lung Transplantation. (2012) 31:797–804. doi: 10.1016/j.healun.2012.03.008 - DOI - PubMed
-
- Burton CM, Iversen M, Carlsen J, Mortensen J, Andersen CB, Steinbrüchel D, et al. . Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J Heart Lung Transplantation. (2009) 28:888–93. doi: 10.1016/j.healun.2009.04.022 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

