Effector Tc17 cells resist shift from OXPHOS to aerobic glycolysis
- PMID: 40453072
- PMCID: PMC12122523
- DOI: 10.3389/fimmu.2025.1571221
Effector Tc17 cells resist shift from OXPHOS to aerobic glycolysis
Abstract
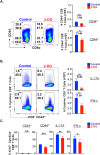
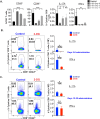
IL-17A-expressing lymphocytes, including Tc17 cells, are instrumental in immunity, immunopathology, and autoimmunity. We have previously shown that experimental attenuated live fungal vaccine-induced Tc17 cells are stable, long-lived without plasticity, and necessary to mediate sterilizing immunity during CD4+ T cell deficiency, which poses higher susceptibility to fungal infections. Cell metabolism is integral for T cell homeostasis but the metabolic adaptations of Tc17 cells are poorly defined. In this study, we hypothesized that effector Tc17 cells adopt high energy-yielding metabolic pathways to form stable, long-lived memory cells in vivo. Using a mouse model of attenuated fungal vaccination, we found that effector Tc17 cells were metabolically highly active with higher proliferation and protein synthesis than IFNγ+ CD8+ T (Tc1) cells. Glucose was necessary for effector Tc17 cell expansion but with less dependency during the late expansion despite the active metabolism. Contrary to established dogma, we found that the effector Tc17 cells preferentially channeled the glucose to OXPHOS than glycolysis, which was correlated with higher mitochondrial mass and membrane potential. Inhibition of OXPHOS shrunk the Tc17 responses while sparing Tc1 cell responses. Tc17 cells actively relied on OXPHOS throughout the expansion period, resisting adaptation to aerobic glycolysis. Our data showed that the effector Tc17 cells predominantly utilize glucose for metabolism through OXPHOS rather than aerobic glycolysis. Our study has implications in vaccine design to enhance the efficacy and immunotherapeutics to modulate the immunity and autoimmunity.
Keywords: CD8+ T cell; OXPHOS; T cell activation; Tc17 cell; antifungal; glycolysis; vaccine responses.
Copyright © 2025 John, Mudalagiriyappa, Chandrashekar and Nanjappa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

