From sensation to regulation: the diverse functions of peripheral sensory nervous system
- PMID: 40453085
- PMCID: PMC12123694
- DOI: 10.3389/fimmu.2025.1575917
From sensation to regulation: the diverse functions of peripheral sensory nervous system
Abstract
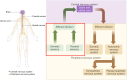
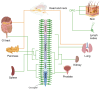
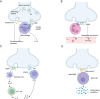
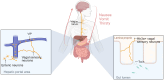
The peripheral sensory nervous system (PNS) has been widely recognized for its role in the collection, processing, and transmission of sensory information, including thermal, mechanical, chemical, and proprioceptive stimuli. In recent years, there has been a growing scholarly interest in the PNS attributable to its multiple physiological and pathophysiological non-sensory roles in the organs it innervates. The PNS exerts regulatory functions within the organs it innervates through direct interactions with local cells or through microbe-nerve-cell interactions that differ from the traditional feedback regulatory modes used by the hormonal and sensory brain-sympathetic/parasympathetic systems. The release of the neuropeptide calcitonin gene related peptide (CGRP) by nerves, through its action on CGRP receptors in peripheral cells, constitutes a primary molecular axis for PNS regulation of organ cells, maintaining tissue homeostasis, facilitating pathological processes, and modulating innate and adaptive immunity. This review highlights the non-sensory functions of the peripheral sensory nervous system in various tissues and organs, focusing on phenotypes, molecular mechanisms and their significance, while also exploring future research directions, methodologies and potential preclinical studies aimed at targeting these pathways for the development of novel therapies.
Keywords: CGRP signaling pathway; microbe-nerve-cell crosstalk; neuro-immune interaction; nonsensory regulatory functions; peripheral sensory nervous system (PNS); sensory signal transduction.
Copyright © 2025 Mei, Zhou, Zhong, Zheng, Deng, Yu and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

