Engineered sequestrins inhibit aggregation of pathogenic alpha-synuclein mutants
- PMID: 40453094
- PMCID: PMC12122515
- DOI: 10.3389/fimmu.2025.1574755
Engineered sequestrins inhibit aggregation of pathogenic alpha-synuclein mutants
Abstract
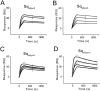
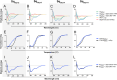
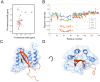
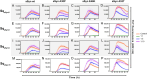
Misfolding and aggregation of the neuronal protein alpha-synuclein (aSyn) has been identified as a hallmark of Parkinson's disease (PD) pathology and other synucleinopathies. Preventing formation of intracellular aSyn accumulations constitutes a therapeutic strategy against disease development. We recently reported on a new type of affinity protein, denoted Sequestrin, aimed for efficient and stable interactions with aggregation-prone amyloidogenic proteins and peptides. Upon binding, sequestrins interact with the aggregation-prone peptide and form a stabilizing four-stranded beta sheet with similarities to the beta sheet rich structures seen in amyloid fibrils. Here, high-affinity aSyn-binding sequestrins were isolated from a large naïve sequestrin library using phage display technology. The best binders demonstrated dissociation constant, KD, values in the 10 nM-range, and structural rearrangements in both the sequestrin and aSyn protein upon binding. Modelling using AlphaFold, followed by NMR spectroscopy suggested that the sequestrins bind an N-terminal region of aSyn that is critical for amyloidogenic aggregation. In an in vitro aggregation study, the sequestrins demonstrated complete inhibition of aSyn aggregation at equimolar concentrations, including the three familial mutants A30P, E46K, and A53T that are associated with Parkinson's disease and Lewy body dementia.
Keywords: Parkinson’s disease; affibody molecule; alpha-synuclein; directed evolution; phage display; sequestrin.
Copyright © 2025 Hjelm, Paslawski, Lendel, Svedmark, Svenningsson, Ståhl, Lindberg and Löfblom.
Conflict of interest statement
HL, SS, and JL are shareholders of Amylonix AB. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

