Improved survival with daratumumab-CyBorD compared with CyBorD as frontline therapy for AL amyloidosis
- PMID: 40453144
- PMCID: PMC12067897
- DOI: 10.1016/j.bneo.2025.100092
Improved survival with daratumumab-CyBorD compared with CyBorD as frontline therapy for AL amyloidosis
Abstract
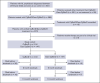
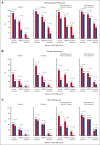
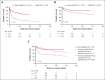
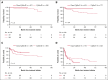
In the ANDROMEDA phase 3 trial, the addition of daratumumab to cyclophosphamide, bortezomib, and dexamethasone (Dara-CyBorD) as frontline therapy significantly improved hematological and organ responses, and event-free survival (EFS) compared with CyBorD. To validate its results, we performed a retrospective study of 361 consecutive patients with newly diagnosed light chain (AL) amyloidosis treated between 2018 and 2022. Patients who received Dara-CyBorD (n = 147) were compared with those treated with CyBorD (n = 214) in key outcome endpoints. The 2-month hematological very good partial response or better rate was higher with Dara-CyBorD than with CyBorD (60.8% vs 31.1%; P < .001). In addition, 2- and 6-month hematological complete response was also higher with Dara-CyBorD (15.3% vs 3.0% and 39.5% vs 17.8%, respectively; both P < .001). Fewer patients treated with Dara-CyBorD required second-line therapy at the 12-month landmark (14.9% vs 42.9%; P < .001). The 6- and 12-month cardiac responses were higher and deeper in the Dara-CyBorD group than in the CyBorD group. Dara-CyBorD was associated with a lower 6-month mortality rate (8.8% vs 16.3%; P = .04) and superior EFS and overall survival (OS). An OS difference between the treatment groups was statistically significant among patients with stage II cardiac disease, and borderline significant for stage IIIA but not for cardiac stage IIIB. In conclusion, the addition of daratumumab to frontline CyBorD significantly improved hematological and organ response rates, reduced early deaths, and prolonged EFS and OS compared with CyBorD.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.A.G. reports honoraria from Celgene, Med Learning Group, Research to Practice, Prothena, Apellis Pharmaceuticals, Amgen, AbbVie, Akcea Therapeutics, Sanofi, Telix Pharmaceuticals, Janssen Oncology, Juno/Celgene, and Alnylam; reports a consulting or advisory role with Prothena and Bristol Myers Squibb/Sanofi; and reports travel, accommodations, and expenses from Prothena, Celgene, and Novartis. A.D. reports a consulting or advisory role with Janssen Research and Development; reports research funding from 10.13039/100006436Celgene (institutional), 10.13039/100005565Janssen Oncology (institutional), 10.13039/100004319Pfizer (institutional), 10.13039/100007723Takeda (institutional), 10.13039/100006400Alnylam (institutional), and Prothena; and reports travel, accommodations, and expenses from Pfizer, Janssen Oncology, and Prothena. P.K. reports honoraria from Pharmacyclics, Sanofi (institutional), BeiGene, MustangBio, AstraZeneca, and AbbVie (institutional); reports consulting or advisory role with Sanofi (institutional); and received research funding from 10.13039/100002429Amgen (institutional), 10.13039/100007723Takeda (institutional), 10.13039/100004339Sanofi (institutional), 10.13039/100006483AbbVie (institutional), 10.13039/100004330GlaxoSmithKline (institutional), Sorrento Therapeutics (institutional), 10.13039/100007136Karyopharm Therapeutics (institutional), 10.13039/100009857Regeneron (institutional), Ichnos Sciences (institutional), 10.13039/100002491Bristol Myers Squibb/10.13039/100006436Celgene (institutional). D.D. reports a consulting role for Alexion (AstraZeneca), Apellis, Bristol Myers Squibb, Genentech/Roche, Janssen, Legend Biotech, Novartis, Ossium Health, Sanofi, and Sorrento Therapeutics; and reports research support from K36 Therapeutics. N.L. reports membership on an entity’s board of directors or advisory committees with Takeda. M.Q.L. reports research funding from 10.13039/100006436Celgene. W.G. reports consulting or an advisory role with Amgen (institutional); reports research funding from 10.13039/501100022241ORIC Pharmaceuticals (institutional) and 10.13039/100001009Bristol Myers Squibb Foundation (institutional); and reports patents, royalties, other intellectual property, with patent number: 10996224 (Assessing and treating precursor plasma cell disorders). T.K. reports research funding from 10.13039/100004336Novartis. J.C. reports stock and other ownership interests in Geron. Y.L. reports consultancy with, and research funding from bluebird bio, 10.13039/100006436Celgene, 10.13039/100005565Janssen, and Kite; reports consultancy with Gamida Cell, 10.13039/100004336Novartis, Juno, Legend, Sorrento, and Vineti; reports research funding from 10.13039/100004334Merck, 10.13039/100007723Takeda, Kite, 10.13039/100004334Merck, 10.13039/100004336Novartis, 10.13039/100004337Roche, and 10.13039/100004339Sanofi; reports membership on an entity’s board of directors or advisory committees with AbbVie, 10.13039/100006436Celgene, 10.13039/100005565Janssen, 10.13039/100007723Takeda, Adaptive, 10.13039/501100004628MedImmune/AstraZeneca; and reports a role on an independent review committee for Oncopeptides. S.V.R. reports honoraria from Research To Practice and Medscape; and receives authorship royalties from Up To Date. S.K.K. reports consulting or advisory role with Takeda (institutional), Janssen Oncology (institutional), Genentech/Roche (institutional), AbbVie (institutional), Bristol Myers Squibb/Celgene (institutional), Pfizer (institutional), Regeneron (institutional), Sanofi (institutional), and K36 (institutional); reports research funding from 10.13039/100007723Takeda (institutional), 10.13039/100006483AbbVie (institutional), 10.13039/100004336Novartis (institutional), 10.13039/100004339Sanofi (institutional), 10.13039/100005565Janssen Oncology (institutional), 10.13039/501100004628MedImmune (institutional), 10.13039/100004337Roche/10.13039/100004328Genentech (institutional), CARsgen Therapeutics (institutional), 10.13039/100024192Allogene Therapeutics (institutional), 10.13039/100004330GlaxoSmithKline (institutional), 10.13039/100009857Regeneron (institutional), and 10.13039/100002491Bristol Myers Squibb/10.13039/100006436Celgene (institutional); and reports travel, accommodations, and expenses from AbbVie and Pfizer. E.M. reports consultation fee from Protego (fee paid to the institution). The remaining authors declare no competing financial interests.
Figures


References
-
- Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. - PubMed
-
- Kastritis E, Fotiou D, Theodorakakou F, et al. Timing and impact of a deep response in the outcome of patients with systemic light chain (AL) amyloidosis. Amyloid. 2021;28(1):3–11. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

